2019 Volume 66 Issue 1 Pages 43-50
2019 Volume 66 Issue 1 Pages 43-50
It is known that long-chain fatty acids bind to free fatty acid receptor 1 (Ffar1), also known as G protein-coupled receptor 40 (GPR40), and amplify glucose-stimulated insulin secretion (GSIS) from pancreatic β-cells and that Ffar1 agonists facilitates insulin secretion and ameliorates glycemic control. On the other hands, pancreatic and duodenal homeobox factor 1 (Pdx1) is an important transcription factor for various β-cell-related genes including insulin gene and thereby contributes to the maintenance of mature β-cell function. The aim of this study was to evaluate how Ffar1 expression in β-cells is altered under diabetic conditions. In this study, we used male obese type 2 diabetic mice and control mice. We evaluated Ffar1 and Pdx1 mRNA and protein expression levels in both mice. In addition, we examined whether Pdx1 is a possible regulator of Ffar1 expression using small interfering RNA for Pdx1 (siPdx1) in β-cell-derived cell line. As the results, Ffar1 mRNA and protein expression in β-cells were significantly lower in obese type 2 diabetic db/db mice compared to control mice which was accompanied by the decreased expression of Pdx1. In addition, down-regulation of Pdx1 expression using siPdx1 suppressed Ffar1 expression. Furthermore, adenoviral Pdx1 overexpression significantly increased Ffar1 expression. In conclusion, Ffar1 expression is markedly down-regulated under diabetic conditions which is accompanied by decreased expression of Pdx1. Furthermore, it is likely that Pdx1 is a regulator of Ffar1 expression in β-cells.
UNDER DIABETIC CONDITIONS, chronic hyperglycemia gradually deteriorates pancreatic β-cell function; insulin biosynthesis and secretion are decreased [1-5]. It is known that long-chain fatty acids bind to free fatty acid receptor 1 (Ffar1), also known as G protein-coupled receptor 40 (GPR40), and amplify glucose-stimulated insulin secretion (GSIS) from β-cells. Ffar1 agonists could facilitate GSIS and lead to amelioration of glycemic control. It was shown that deletion of Ffar1 impaired GSIS in vivo in mice and that overexpression of Ffar1 in β-cells augmented GSIS and improved glucose tolerance [6, 7]. Therefore, Ffar1 agonists would be promising drugs for type 2 diabetes mellitus. On the other hands, Pdx1 is a very important transcription factor for insulin gene and various other β-cell-related genes and thereby contributes to the maintenance of mature β-cell function [8-14]. In this study, we evaluated how Ffar1 expression in β-cells was altered under diabetic conditions.
We used male obese type 2 diabetic C57/BsJ-db/db mice (7, 10 and 14 weeks of age) and male control non-diabetic m/m mice (14 weeks of age) (Clea, Tokyo, Japan). They were housed two to three animals per cage in all experiments under controlled ambient conditions and a 14:10 h light/dark cycle with lights on at 7 a.m. Animals were given free access to drinking water and food and were maintained at 25°C. This study was approved by the Animal Use Committee of Kawasaki Medical School (No. 17-16) and was conducted in compliance with the Animal Use Guidelines of the Kawasaki Medical School.
Islet isolationIslets were isolated after clamping the common bile duct at its entrance to the duodenum, 2 mL of Hanks’ balanced salts solution (HBSS) (Sigma) containing 1.28 mg of collagenase (Sigma, C9263-500MG) per mL was injected into the duct. The swollen pancreas was surgically removed and incubated at 37°C for 15 min. Thereafter, 12 mL of cold HBSS containing 9% newborn calf serum (NCS) was added to stop the digestion reaction at shaking 20 times and standing for 5 min on ice. Digested pancreas was dispersed by pipetting and rinsed twice with 9 mL of ice-cold HBSS. The digested tissue was re-suspended in cold RPMI medium containing 10% fetal bovine serum (FBS). Islets were manually picked under a dissection microscope using a pipette.
Real-time RT-PCRRNA samples from the isolated islets were extracted using an RNeasy Mini Kit (Cat. No. 74106, QIAGEN). The primers were designed using Primer Express, based on mRNA sequences downloaded from the GenBank nucleotide database. Primer sequences were as follows: mouse Pdx1 (forward) CGGCTGAGCAAGCTAAGGTT, (reverse) TGGAAGAAGCGCTCTCTTTGA; mouse Ffar1 (forward) CGCTGGGCTTTCCATTGA, (reverse) GCTGGGAGTGAGTCGCAGTT. A reaction mixture was prepared by combining 1 μL of sample, 1 μL of 50 nM primers, 12.5 μL of Sybr Green PCR Master Mix (Applied Biosystems) solution, and 10.5 μL of diluent solution. Dissociation curve analysis was performed in all experiments to determine the dissociation temperature, and the size of the PCR products was confirmed using agarose gel electrophoresis. Ffar1 and Pdx1 mRNA levels were normalized by 36B mRNA level in each experiment.
ImmunostainingThe sections were incubated with each primary antibody: anti-Ffar1 antibody (Bioss, No. bs-9564R), anti-Pdx1 antibody (Cell Signaling, No. 5679) and anti-insulin antibody (abcam, No. ab7842). Goat anti-guinea pig IgG (Alexa Fluor 594 conjugate) (Invitrogen, No. A-11076) and donkey anti-rabbit IgG (Alexa Fluor 488 conjugate) (Invitrogen, No. A-21206) were used as secondary antibodies.
Western blottingAnti-Ffar1 antibody (Bioss, No. bs-9564R) and anti-Pdx1 antibody (Cell Signaling, No. 5679) were used as primary antibodies. Donkey anti-rabbit IgG (Amersham, No. NA934VS) was used as a secondary antibody. Pdx1 and Ffar1 protein levels were normalized by β-actin protein level in each experiment.
Cell culture and transfection of small interfering RNA (siRNA) directed to Pdx1 (siPdx1)Pancreatic β-cell-line MIN6 cells were cultured in Dulbecco’s Modified Eagle’s Medium (15% FBS, 1% Penicillin/Streptomycin) (Sigma). In transfection of siPdx1, Lipofectamine RNAiMAX Reagent (Invitrogen) and Opti-MEM I Reduced Serum Medium (Gibco) were used. Two days after transfection of siPdx1, we confirmed that Pdx1 expression level was down-regulated and examined whether Ffar1 expression level was altered in MIN6 cells.
Preparation of Pdx1 expressing adenovirus (Ad-Pdx1) and control adenovirus (Ad-GFP)We prepared recombinant adenovirus expressing Pdx1 (Ad-Pdx1) with the AdEasy system (Quantum, Montreal, Canada). First, we cloned the encoding region of Pdx1 into a shuttle vector pAdTrack-CMV. Next, to produce homologous recombination, we introduced linearized plasmid containing Pdx1 and adenoviral backbone plasmid pAdEasy-1 into electrocompetent E. coli BJ5183 cells by electroporation (2,500 V, 200 Ohms, 25 μFD). The resultant plasmids were then re-transformed into E. coli XL-Gold Ultracompetent Cells (Stratagene, La Jolla, CA). Finally, the plasmids were linearized with PacI and then transfected into the adenovirus packaging cell line 293 using LipofectAMINE (Invitrogen, Carlsbad, CA). Ten days after transfection, the cell lysate was collected from the 293 cells. The cell lysate was added to fresh 293 cells and when most of the cells were killed by the adenovirus infection and detached, the cell lysate was obtained again (this process was repeated three times). The control adenovirus expressing green fluorescent protein (Ad-GFP) was prepared in the same manner. Two days after infection of Ad-Pdx1 or Ad-GFP, we confirmed that the adenovirus was actually infected in HeLa cells by checking GFP expression through fluorescence microscope. Next, we confirmed that Pdx1 expression level was augmented and examined whether Ffar1 expression level was altered by Pdx1 overexpression in HeLa cells. This study was approved by the Recombinant DNA Experiment Committee (No. 16-50-1) and the Biosafety Committee in Kawasaki Medical School (No. 16012-1).
Experiments with SGLT2 inhibitor luseogliflozin10-week-old male C57/BsJ-db/db mice were treated withSGLT2 inhibitor luseogliflozin 0.01% (Taisho Pharmaceutical Co., Ltd) or vehicle (0.05% carboxymethylcellulose, oral) for 4 weeks. It is noted here that all samples with SGLT2 inhibitor luseogliflozin were those obtained in the previous experiments (Okauchi S et al. Biochem Biophys Res Commun 470: 772–782, 2016) [15]. Therefore, please refer to this manuscript about the detailed information of these experiments.
To examine the alteration of Ffar1 expression under diabetic conditions, we performed an immunostaining for Ffar1 in obese type 2 diabetic db/db mice at 7, 10, 14 weeks of age. As shown in Fig. 1A, Ffar1 expression in β-cells in diabetic db/db mice at 10 and 14 weeks of age was lower compared to that in 7-week-old db/db mice. Pdx1 expression in β-cells in diabetic db/db mice was at 10 and 14 weeks of age also lower compared to that in 7-week-old db/db mice.
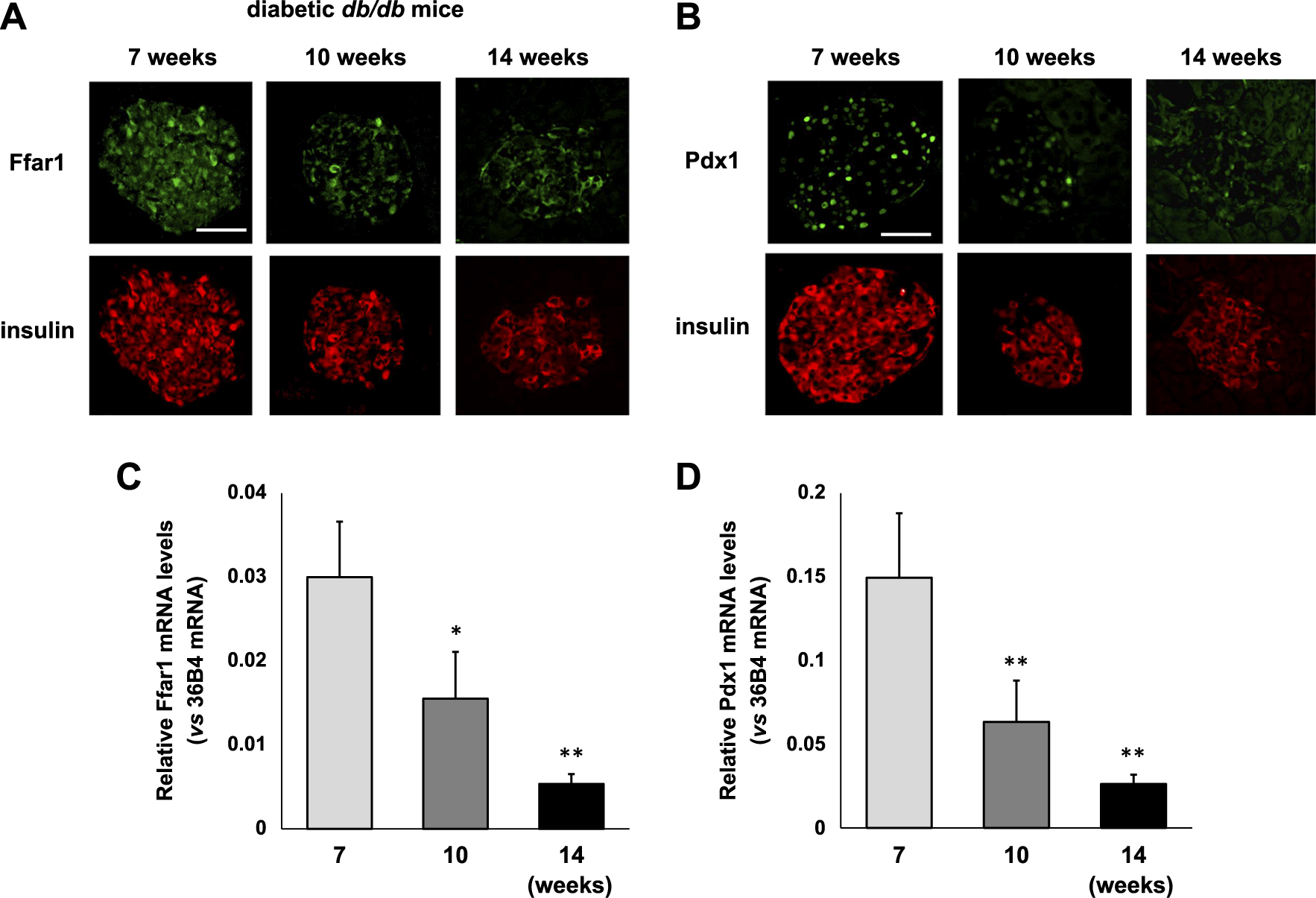
(A) Immunostaining for Ffar1 and insulin in obese diabetic db/db mice at 7, 10 and 14 weeks of age. Each panel shows a representative picture in both mice (n = 4). Ffar1 expression in β-cells in diabetic db/db mice is gradually decreased under diabetic conditions. Bar 50 μm. (B) Immunostaining for Pdx1 and insulin in obese diabetic db/db mice at 7, 10 and 14 weeks of age. Each panel shows a representative picture in both mice (n = 4). Pdx1 expression in β-cells in diabetic db/db mice is also gradually decreased under diabetic conditions. Bar 50 μm. (C, D) Ffar1 (C) and Pdx1 mRNA expression (D) in obese diabetic db/db mice at 7, 10 and 14 weeks of age. Ffar1 and Pdx1 mRNA levels are normalized by 36B mRNA level. Data are presented as mean ± SD (n = 5–8). **:p < 0.001, *:p < 0.005 (vs. 7-week-old db/db mice).
Next, to quantitatively examine the alteration of Ffar1 mRNA expression levels under diabetic conditions, we examined Ffar1 mRNA expression levels in diabetic db/db mice at 7, 10, 14 weeks of age. As shown in Fig. 1C, Ffar1 mRNA expression levels in islets isolated from 10-week-old diabetic db/db mice were significantly lower compared to those from 7-week-old diabetic db/db mice (p < 0.005). Ffar expression levels were more markedly lower in 14-week-old db/db mice. Pdx1 mRNA expression levels in islets isolated from 10-week-old diabetic db/db mice were also significantly lower compared to those from 7-week-old diabetic db/db mice (p < 0.001). Pdx1 expression levels were also more markedly lower in 14-week-old db/db mice.
Suppression of Pdx1 expression level with siPdx1 leads to the down-regulation of Ffar1 mRNA and protein expression levels in pancreatic β-cell line MIN6 cellsTo examine whether Pdx1 is a potential regulator of Ffar1 expression in β-cells, we prepared small interfering RNA (siRNA) directed to Pdx1 (siPdx1) and exposed MIN6 cells to siPdx1 or scrambled control siRNA. As shown in Fig. 2A, Pdx1 mRNA levels were significantly suppressed after the treatment with siPdx1 compared to control siRNA (p < 0.01). In western blotting, the significant reduction of Pdx1 protein levels was also confirmed after the treatment with siPdx1 (p < 0.01) (Fig. 2B). Such reduction of Pdx1 expression levels led to the significant down-regulation of Ffar1 mRNA expression levels (p < 0.01) (Fig. 2C). Ffar1 protein expression was also significantly decreased by the reduction of Pdx1 expression (p < 0.05) (Fig. 2D). These results suggest that Pdx1 plays a potential role in the regulation of Ffar1 expression in pancreatic β-cells.
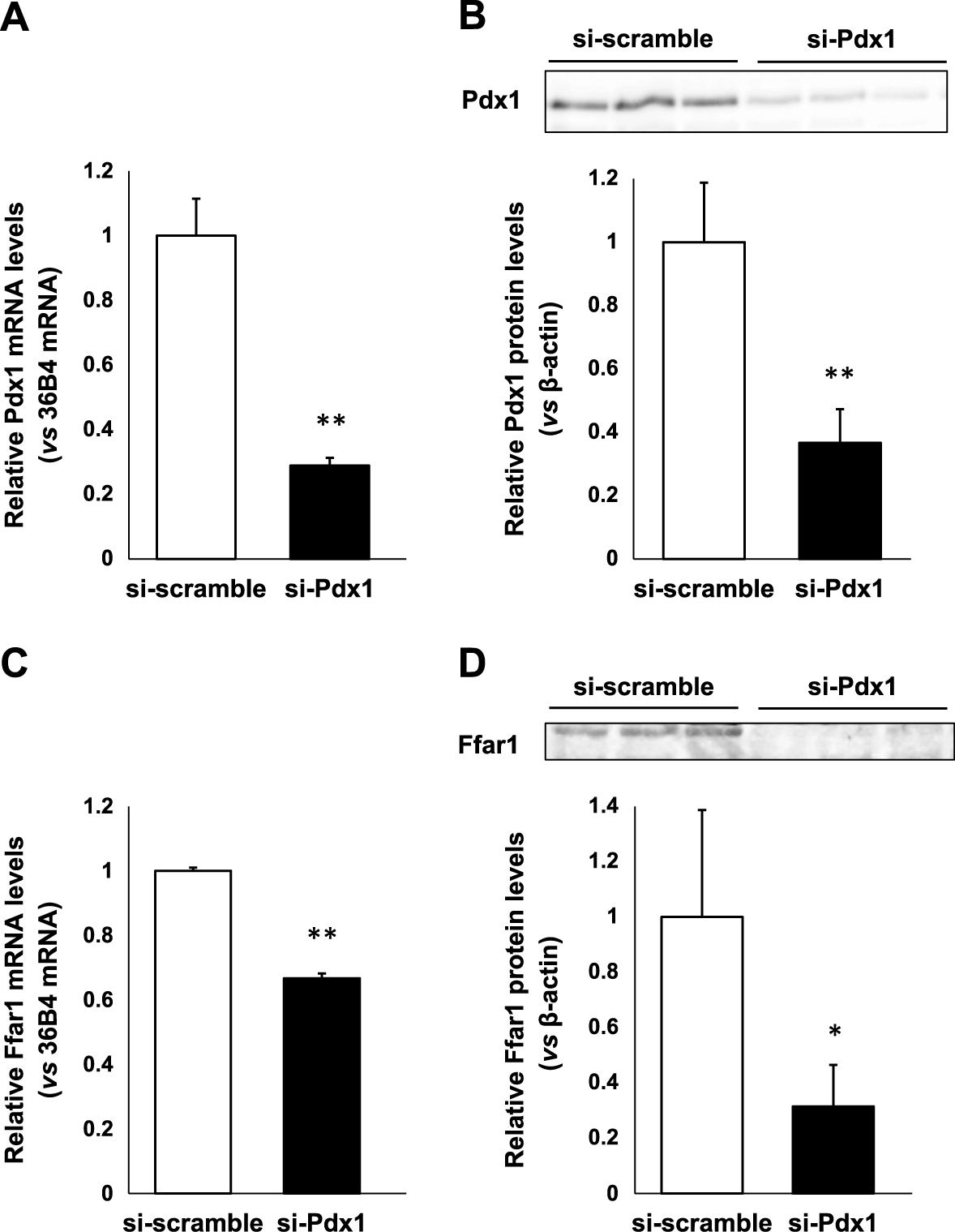
Effects of siPdx1-mediated suppression of Pdx1 expression on Ffar1 expression in pancreatic β-cell line MIN6 cells. (A, B) Pdx1 mRNA and protein expression levels after the treatment with siPdx1. (C, D) Ffar1 mRNA and protein expression levels after the treatment with siPdx1. Pdx1 and Ffar1 mRNA and protein levels are normalized by 36B mRNA and β-actin protein level, respectively. Data are presented as mean ± SD (A, C: n = 4) (B, D: n = 3). **: p < 0.01, *: p < 0.05.
In order to demonstrate the working hypothesis that Pdx1 regulates Ffar1 expression, we prepared Pdx1 expressing adenovirus (Ad-Pdx1) and control adenovirus (Ad-GFP) and exposed them to HeLa cells which have few endogenous Pdx1 expression. Fig. 3A shows subcellular localization of Pdx1-GFP after the adenoviral infection. As shown in Fig. 3B, Pdx1 mRNA levels were significantly increased after the treatment with Ad-Pdx1 compared to control Ad-GFP (p < 0.001). In western blotting, the significant increase of Pdx1 protein levels was also confirmed after the treatment with Ad-Pdx1 (p < 0.005) (Fig. 3C). Such increase of Pdx1 expression levels led to the significant augmentation of Ffar1 mRNA expression levels (p < 0.001) (Fig. 3D). Ffar1 protein expression was also significantly increased by adenoviral Pdx1 overexpression (p < 0.001) (Fig. 3E). These results suggest the possibility that Pdx1 plays a role in the regulation of Ffar1 expression. It is noted, however, that Pdx1 and Ffar1 mRNA levels in HeLa cells are very low compared to β-cells. Therefore, further experiments would be necessary in order to strengthen such working hypothesis.
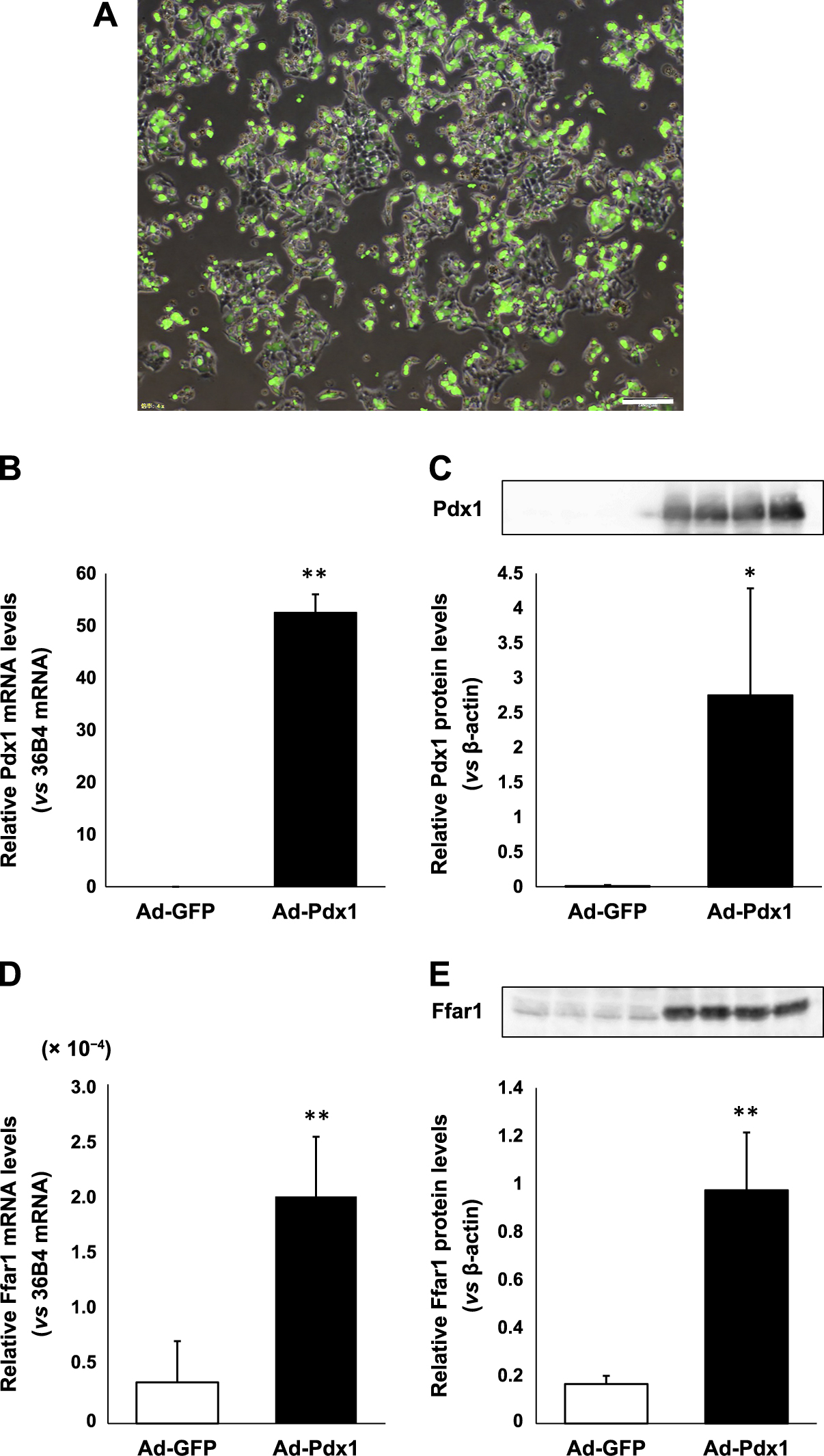
Effects of adenoviral overexpression of Pdx1 levels on Ffar1 expression in HeLa cells. (A) Subcellular localization of Pdx1-GFP in HeLa cells. Bar, 200 μm. (B, C) Pdx1 mRNA and protein expression levels after the treatment with Ad-Pdx1. (D, E) Ffar1 mRNA and protein expression levels in HeLa cells after the treatment with Ad-Pdx1. Pdx1 and Ffar1 mRNA and protein levels are normalized by 36B mRNA and β-actin protein level, respectively. Data are presented as mean ± SD (B, D: n = 12) (C, E: n = 4). **: p < 0.001, *: p < 0.005.
To examine the alteration of Ffar1 expression after amelioration of glycemic control, we performed an immunostaining for Ffar1 in control m/m mice (14 weeks of age) and obese type 2 diabetic db/db mice (14 weeks of age) with and without treatment of SGLT2 inhibitor luseogliflozin (0.01% in chow) for 4 weeks (from 10 to 14 weeks of age). As shown in Fig. 4A, Ffar1 expression in β-cells in diabetic db/db mice was markedly lower compared to that in control mice. However, Ffar1 expression in β-cells in diabetic db/db mice after SGLT2 inhibitor treatment was restored compared to that without treatment. As shown in Fig. 4B, Pdx1 expression in β-cells in diabetic db/db mice was markedly lower compared to that in control mice. However, Pdx1 expression in β-cells in diabetic db/db mice after SGLT2 inhibitor treatment was also restored compared to that without treatment.

(A) Immunostaining for Ffar1 and insulin in control m/m mice (14 weeks of age) and obese diabetic db/db mice (14 weeks of age) with and without treatment of SGLT2 inhibitor luseogliflozin. Each panel shows a representative picture in both mice. Ffar1 expression in β-cells in diabetic mice is markedly lower compared to that in control mice. However, Ffar1 expression in β-cells after treatment with SGLT2 inhibitor is markedly higher compared to that without such treatment. (B) Immunostaining for Pdx1 and insulin in control m/m mice and obese diabetic db/db mice with and without treatment of SGLT2 inhibitor leuseogliflozin. Each panel shows a representative picture in both mice. Pdx1 expression in β-cells in diabetic mice is markedly lower compared to that in control mice. However, Pdx1 expression in β-cells after treatment with SGLT2 inhibitor is also higher compared to that without such treatment. (C, D) Ffar1 (C) and Pdx1 mRNA expression (D) in control m/m mice and obese diabetic db/db mice with and without treatment of SGLT2 inhibitor luseogliflozin. Ffar1 and Pdx1 mRNA levels are normalized by 36B mRNA level. Data are presented as mean ± SD (n = 4–8). **: p < 0.001, *: p < 0.005. It is noted here that all samples in Fig. 4 were those obtained in the previous experiments (Okauchi S et al. Biochem Biophys Res Commun 470: 772–782, 2016) [15]. Therefore, please read this manuscript about the detailed information of these experiments.
Next, to quantitatively examine the alteration of Ffar1 mRNA expression levels after amelioration of glycemic control, we examined Ffar1 mRNA expression levels in control m/m mice and diabetic db/db mice with and without SGLT2 inhibitor treatment. As shown in Fig. 4C, Ffar1 mRNA expression levels in diabetic db/db mice was significantly lower compared to that in control m/m mice (p < 0.001). However, Ffar1 mRNA expression levels in islets isolated from diabetic db/db mice after SGLT2 inhibitor were restored compared to those without treatment (p < 0.001). As shown in Fig. 4D, Pdx1 mRNA expression levels in diabetic db/db mice was also significantly lower compared to that in control m/m mice (p < 0.005). However, Pdx1 mRNA expression levels in islets isolated from diabetic db/db mice after SGLT2 inhibitor were also restored compared to those without treatment, although it did not reach a statistical significance. It is noted here that all samples in Fig. 4 were those obtained in the previous experiments (Okauchi S et al. Biochem Biophys Res Commun 470: 772–782, 2016) [15]. Therefore, please refer to this manuscript about the detailed information of these experiments.
In this study, we showed that Ffar1 expression in pancreatic β-cells was significantly decreased in obese type 2 diabetic db/db mice which was accompanied by the decreased expression of Pdx1 (Fig. 1). In addition, we showed that suppression of Pdx1 expression level with siPdx1 led to the down-regulation of Ffar1 mRNA and protein expression levels in MIN6 cells (Fig. 2). Furthermore, we prepared Pdx1 expressing adenovirus and showed that adenoviral overexpression of Pdx1 led to the augmentation of Ffar1 mRNA and protein expression levels (Fig. 3). Furthermore, Ffar1 expression levels were restored by the amelioration of glycemic control with SGLT2 inhibitor luseogliflozin which was accompanied by restoration of Pdx1 expression (Fig. 4). These data suggest that Pdx1 is involved in the regulation Ffar1 expression as well as other β-cell-related factors. We do not think that Pdx1 is the only regulator of Ffar1 expression but it is likely that Pdx1 is at least one of the important regulators of Ffar1 expression.
It is well known that Ffar1 is involved in glucose-stimulated insulin secretion (GSIS) from β-cells, and thus we think that such down-regulation Ffar1 expression could lead to reduction of GSIS and thus explain, at least in part, the molecular mechanism for pancreatic β-cell dysfunction (pancreatic β-cell glucose insensitivity) found in type 2 diabetes. We think that it is very important to preserve Pdx1 and/or Ffar1 expression level for a long period of time. In addition, it is possible such preservation lead to, at least in part, protect β-cells against glucose insensitivity. Since it is known that Ffar1 is mainly involved in the maintenance of glucose response of β-cells [16], without additional experiments we cannot exclude the possibility that β-cell destruction is not rescued by Ffar1 restoration alone. However, if we could find out the factor and/or compound which can directly increase Pdx1 and/or Ffar1 expression in β-cells, it would be helpful for the preservation of β-cell function. In addition, the information obtained in this study would be useful when Ffar1 agonists are developed as a potential drug for the treatment of type 2 diabetes and when they are applied to clinical practice. For example, considered from the fact that Ffar1 expression is down-regulated under diabetic conditions, Ffar1 agonists would be useful at an earlier stage of diabetes compared to advanced stage.
In conclusion, Ffar1 mRNA and protein expression are markedly down-regulated under diabetic conditions accompanied by decreased expression of Pdx1. Such phenomena are likely involved in β-cell glucose insensitivity found in subjects with type 2 diabetes. In addition, it is likely that Pdx1 is involved in the regulation of Ffar1 expression in β-cells.
We thank Ms. Yuka Nogami for her technical assistance.
Kohei Kaku has been an advisor to, received honoraria for lectures from, and received scholarship grants from Novo Nordisk Pharma, Sanwa Kagaku Kenkyusho, Takeda, Taisho Pharmaceutical Co., MSD, Kowa, Sumitomo Dainippon Pharma, Novartis, Mitsubishi Tanabe Pharma, AstraZeneca, Nippon Boehringer Ingelheim Co., Chugai, Daiichi Sankyo, and Sanofi.
Hideaki Kaneto has received honoraria for lectures and received scholarship grants from Sanofi, Novo Nordisk, Lilly, Boehringer Ingelheim, MSD, Takeda, Ono Pharma, Daiichi Sankyo, Sumitomo Dainippon Pharma, Mitsubishi Tanabe Pharma, Pfizer, Kissei Pharma, AstraZeneca, Astellas, Novartis, Kowa, Chugai and Taisho Pharma.
This work was supported by a Grant-in-Aid from the Japan Society for the Promotion of Science (No. 16K09770 to H.K.) and Research Project Grants from the Kawasaki Medical School (No 26-12 to H.K.).
This article does not contain any studies with human subjects performed by the any of the authors.