2017 Volume 63 Issue 2 Pages 32-38
2017 Volume 63 Issue 2 Pages 32-38
Sleep-disordered breathing (SDB) is frequently observed in patients with heart failure (HF), and complex pathologic conditions exist between both conditions. In this review article, we describe the characteristics of SDB complicated with HF, the prognostic impact of SDB in HF patients, and the favorable effects of positive airway pressure in HF patients with SDB.
Heart failure (HF) is a systemic disease with a devastating prognosis, and affects many of the organ systems. In HF patients, sleep-disordered breathing (SDB) is a frequent co-morbidity and its prevalence is related to the severity of the clinical status1-4). Over 50% of patients with HF (with either preserved or reduced ejection fraction [EF]) have SDB, which is around 10 times the rate in the general population2). In current clinical practice, SDB remains undiagnosed in many HF patients. Older age, male gender, increased body mass index, lower EF, and the presence of atrial fibrillation are independent predictors for the presence of SDB3). Referring to recent articles1-4), and adding our findings, we describe the characteristics of SDB complicated with HF, the prognostic impact of SDB in HF patients, and the favorable effects of positive airway pressure in HF patients with SDB.
Polysomnography including the assessment of the electroencephalogram, electrooculogram, electrocardiogram, electromyogram, nasal and oral airflows, respiratory movement (thoracic and abdominal respiratory effort), snoring oxygen saturation, body position, and sleep stage, has long been considered the gold standard test for SDB (Figure 1). An apnea is the absence of inspiratory airflow for at least 10 sec. A hypopnea is a lesser decrease in airflow, lasting 10 sec or longer, and associated with a drop in arterial oxygen saturation and/or an electroencephalographic arousal1-4). Apnea and hypopnea are classified as obstructive or central, but in either case, they result from an absence or reduction of breathing command of brainstem to upper airway muscles (e.g., genioglossus) and/or lower thoracic inspiratory pump muscles (diaphragm and intercostal muscles)1,5). SDB includes obstructive sleep apnea (OSA), central sleep apnea (CSA) with Cheyne-Stokes respiration (CSR), or a combination of both. The pattern of neural output determines the phenotype. OSA is characterized by cessation or marked reduction of the airflow in the presence of respiratory effort (Figure 1). OSA occurs when complete upper airway occlusion occurs (absent airflow, tongue falling back, regardless of activity of the inspiratory thoracic pump muscles. In OSA, there is collapse of the pharynx during sleep with consequent upper airway obstruction, often with snoring. Predisposing factors include obesity, a short neck, and retrognathism. In contrast, CSA is characterized by cessation of both airflow and respiratory effort during sleep (Figure 2). CSA occurs when there is a transient reduction by the pontomedullary pacemaker in the generation of breathing rhythm, usually reflecting changes in the partial pressure of CO2, which can fall below the apnea threshold1). CSR is one type of CSA, and is recognized as a repeated respiratory pattern of gradually increasing and decreasing ventilatory volume.
In HF, rostral fluid shift during sleep leads to pharyngeal edema, which may exacerbate the tendency to obstruct6). In CSA, the underlying abnormality is in the regulation of breathing in the respiratory centers of the brainstem. In normal physiology, minute ventilation during sleep is primarily regulated by chemoreceptors in the brain stem and carotid bodies, which trigger an increase in respiratory drive in response to a rise in arterial carbon dioxide (PaCO2), thus maintaining PaCO2 within a narrow range2,6). Patients with HF and CSA tend to have an exaggerated respiratory response to carbon dioxide associated with excess sympathetic nervous activity, and so that a modest rise in PaCO2 that may occur during sleep results in inappropriate hyperventilation2,7,8). This drives the PaCO2 below the ‘apneic threshold’, at which point the neural drive to respire is too low to stimulate effective inspiration, and an apnea or hypopnea ensues.
Whilst polysomnography provides comprehensive data, it is expensive, laborious, and not available in all centers. A more limited, multichannel sleep polygraphy (with oxygen saturation, nasal airflow, and chest and abdominal movement recording capability) is more widely available and can be set up by the patient at home. Studies comparing the diagnostic accuracy of home polygraphy have shown that it has a sensitivity and specificity of 90-100% for the diagnosis of significant SDB2,9,10). Because of the lack of subjects’ and physicians’ awareness of SDB, especially in subjects with HF, and limited access to a portable sleep monitor or overnight polysomnography, the majority of SDB subjects remain undiagnosed. SDB is associated with an altered sympathovagal balance determined by the nocturnal cyclic alternating of apneas and hyperventilation-bradycardia during apnea, followed by abrupt tachycardia11). This phenomenon causes cyclic variation in heart rate11). Not only OSA but also CSA-CSR demonstrated heart rate oscillations12). Both types of SDB present cyclic lengthening/shortening in the R-R interval, during apnea-post apneic hyperventilation13). We previously reported that cyclic variation of heart rate score (CVHRS) determined by Holter electrocardiogram is a useful screening index for severe SDB in HF subjects14). In that study, there was a significant positive correlation between CVHRS and apnea hypopnea index, which is a primary index of SDB (R=0.60, P<0.001)14). In addition, the receiver operating curve analysis revealed that CVHRS (a cut off value of 30/h) identified severe SDB with a sensitivity of 82%, specificity of 77%, and area under the curve of 0.83 (95% confidence interval 0.72-0.93)14). Pacemaker algorithms were recently developed to detect and quantify SDB accurately15). It is now possible to measure thoracic impedance continually between the right ventricular lead tip and the generator. On inspiration, the increased volume of air in the chest increases thoracic impedance, with the inverse occurring on expiration, with consequent proportional changes in detected potential difference. It has been recently reported that intrathoracic impedance has a sensitivity of 88.9% and specificity of 84.6% for the diagnosis of moderate to severe SDB15).

Obstructive sleep apnea
Obstructive sleep apnea (downward arrow) is characterized by cessation or marked reduction of the ariflow (airflow band) in the presence of respiratory effort (thorax and abdomen band).

Central sleep apnea
Central sleep apnea (downward arrow) is characterized by cessation of both ariflow (airflow band) and respiratory effort (thorax and abdomen band) during sleep. Cheyne-Stokes respiration is recognized as increasing and decreasing gradually repeated respiratory pattern.
Inspiratory efforts in OSA against the occluded upper airway are associated with intrathoracic pressure oscillations that result in increased sympathetic activity4,16). The hypoxia, hypercapnia, and arousal from sleep that occur at the end of the OSA further increase sympathetic activity. The post-apneic period is when a patient recovers upper airway patency and is often characterized by marked increases in blood pressure and heart rate4,17). Importantly, the adverse cardiovascular consequences of OSA are not confined to sleep. Indeed, increased daytime sympathetic nervous activity and arterial hypertension are also reported to occur in OSA patients. OSA may accelerate the progression of HF in several ways. The negative intrathoracic pressure generated by the respiratory muscles trying to inspire against the closed airway increases venous return to the right heart;thus, increasing pre-load and causing the septum to shift to the left, which may compromise left ventricular (LV) function2-4). The ability of the failing left ventricle to cope with enhanced preload is further impaired by the increased transmural pressure during episodes of negative intrathoracic pressure, which in turn increases the afterload. Apnea and hypopnea activate the sympathetic nervous system, and levels of circulating catecholamines and muscle sympathetic nervous activity are higher in those with SDB and HF than HF without SDB2,8,18). These factors accompanied with inflammatory mediators cause hypertension, arrhythmia, coronary arterial disease, myocardial dysfunction and HF4). We have previously reported that SDB is associated with latent myocardial damage and alteration of myocardial carnitine metabolism in patients with HF, presented by higher circulation troponin T and carnitine levels19). In addition, SDB induces impairment of vagal activity, cardiac electrical instability, and ventricular arrhythmias across a 24-hour period accessed by heart rate variability and heart rate turbulence using Holter electrocardiogram20,21). It still remains unclear whether CSA-CSR is merely a marker of the severity of HF, or an important risk factor that independently worsens the prognosis of HF patients, and whether treatment of CSA-CSR is useful in HF patients. When multivariate analyses were performed to control for potential confounders involved in determining outcome in patients with HF, CSA was an independent factor for death or cardiac transplantation in these patients4,22,23). Some large-scale studies have demonstrated that SDB is associated with occurrence of ventricular arrhythmias20,24) and adverse prognosis in HF patients25,26).
Continuous positive airway pressure (CPAP) is widely established in clinical guidelines for the treatment of symptomatic OSA in the non-HF population5). CPAP provides continuous pressure throughout the respiratory cycle. The resultant positive pressure prevents the pharynx from collapsing and thus improves apnea and hypopnea (Figure 3). It may have additional benefits in HF, as positive end-expiratory pressure prevents alveoli collapsing secondary to pulmonary edema and maintains alveoli at a greater diameter, thus reducing the work of breathing (Figure 3). It also increases alveolar recruitment, improves gas exchange, and reduces right to left intrapulmonary shunting of blood1,2). The positive intrathoracic pressure reduces venous return (preload) and LV transmural pressure (afterload), and may therefore benefit cardiac function in some patients2). In the current study, CPAP caused abolition of negative intrathoracic pressure swings and reductions in nocturnal blood pressure, which caused a dramatic reduction in LV afterload that was accompanied by a decrease in heart rate. We reported that CPAP improves right ventricular systolic function, pulmonary function and exercise capacity, resulting in reduction in all-cause mortality in HF patients with preserved EF27).
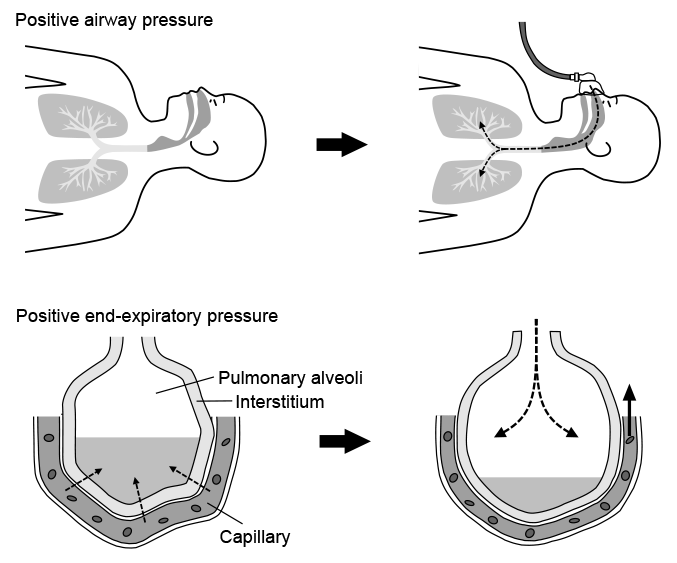
Effect of positive airway pressure
Upper panels: Positive airway pressure widens an upper airway and pulmonary alveoli.
Lower panels: Positive end-expiratory pressure expands pulmonary alveoli, decreases pulmonary fluid and improves congestion and gas exchange in patients with heart failure.
The optimal management of CSA in HF is less well determined than that of OSA. Standard therapy of HF such as diuretics28), beta-blockers29), and cardiac resynchronization therapy30) improves CSA in HF patients. Therefore, standard HF therapy is performed prior to specific CSA therapy in HF. Nocturnal oxygen therapy has been shown to reduce sympathetic drive and increase nocturnal oxygen saturation in HF patients with CSA31). Oxygen therapy improves not only CSA, but also attenuates sympathetic nervous activity and ongoing myocardial damage32), and improve LVEF at least up to 12 weeks33). Nasal CPAP treatment showed an improvement in the CSA, an increase in LVEF and nocturnal oxygen saturation, a reduction in the plasma levels of norepinephrine, and an improved 6-min walking distance compared with the placebo group34). Unfortunately, no improvement was found in the overall death and heart transplantation rates between the two groups. However, a post-hoc analysis showed a decrease in mortality in patients in whom CPAP therapy resulted in improvement of CSA35). Interestingly, the responder group (CPAP-CSA-suppressed) had a significant increase in LVEF at 3 months, and had a higher transplantation-free survival than the control subjects35). No differences in any of these variables were found in the non-responder group (CPAP-CSA-unsuppressed)35). Therefore, suppression of CSA has been focused upon. Technological progress has led to the development of devices for adaptive servo ventilation (ASV), which provide varying amounts of ventilatory pressure support against a background of low-level CPAP36). Several studies suggest that ASV is more effective than CPAP, bi-level pressure support ventilation, or increased dead space in alleviating CSA36-39). We demonstrated that ASV improves not only left ventricular systolic40-42) and diastolic function43), but also pulmonary function27), renal function44), vascular function43), as well as prognosis in HF patients with reduced or preserved EF27,40-44). Recent meta-analyses on ASV in HF patients with CSA suggested an overall improvement in CSA, as well as improvements in LVEF, diastolic dimensions and function, 6-min walk test distance, plasma natriuretic peptide concentration, and sympathetic activity2,45). However, a recent randomized trial failed to demonstrate that ASV improves prognosis of HF patients with CSA46). Further studies are needed to determine whether managing SDB improves prognosis of HF patients.
The authors would like to acknowledge professor Takeishi and professor Maruyama for their instructions, support and valuable advice. The authors would also like to thank all those who contributed to these studies.