Abstract
Background:Laparoscopic and endoscopic cooperative surgery (LECS) is a well-recognized surgical procedure for gastric gastrointestinal stromal tumor (GIST). In this report, we describe the clinical outcomes of LECS procedures for gastric GIST in our institution.
Methods:We performed LECS procedures, including classical LECS, inverted LECS, closed LECS, and combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique (CLEAN-NET), in 40 gastric intraluminal and intramural type GIST patients, whose tumors were ≤ 50 mm in diameter, between September 2012 and December 2020. The patient background, surgical outcomes, postoperative morbidity and mortality, as well as the tumors’ clinicopathological characteristics were analyzed retrospectively.
Results:Pathological findings showed that most patients had a low or very low risk of tumor recurrence, while one patient had a high risk according to the modified-Fletcher’s classification. The median length of postoperative hospital stay was 7 days. Only one patient had severe postoperative grade III complications according to the Clavien-Dindo (C-D) classification, after closed LECS, but was treated successfully with endoscopic hemostasis for postoperative hemorrhage. The remaining patients treated with LECS did not have severe complications. During the follow-up period (median, 31 months), all patients were disease-free, with no tumor recurrence or metastases.
Conclusion:LECS is a safe surgical procedure for gastric intraluminal and intramural type GIST ≤ 50 mm in diameter, with good clinical outcomes.
Introduction
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal subepithelial tumor with malignant potential in the stomach. Once a gastric subepithelial tumor is diagnosed as GIST, complete tumor resection should be considered1). Since GIST rarely metastasizes to the lymph nodes, partial or wedge resection of the stomach wall containing the subepithelial tumor is considered curative treatment.
According to the clinical practice guidelines for GIST in Japan, laparoscopic resection as minimal invasive surgery is acceptable for gastric GIST that are ≤ 50 mm in diameter2). To prevent gastric wall deformity and preserve gastric function, laparoscopic and endoscopic cooperative surgery (LECS) for gastrointestinal tumors, named ‘classical LECS,’ was first reported in 20083). Furthermore, a modified LECS procedure, named ‘inverted LECS,’ which avoids the contamination of gastric juice and the seeding of tumor cells into the abdominal cavity, was reported in 20124). Since 2016, we have mainly performed inverted LECS for gastric GIST in our hospital and employed CLEAN-NET5) or closed LECS6) as a non-exposure technique for GIST with delle, i.e., an ulcer on the mucosal surface of the submucosal tumor, which has the potential to induce peritoneal seeding of tumor cells.
While the safety and usefulness of LECS procedures for gastric GIST have been described in previous reports7,8), there has not been sufficient discussion of intra-abdominal abscesses or recurrence associated with the dissemination of gastric contents or tumor cells in patients who have undergone LECS. Therefore, in the present report, we describe the clinical outcomes of LECS procedures for gastric GIST, including postoperative complications and recurrence, in a consecutive series of 40 patients treated at our institution.
Materials and methods
Patients
Between September 2012 and December 2020, a total of 40 patients with gastric GIST underwent LECS at the Department of Gastrointestinal Tract Surgery, Fukushima Medical University Hospital (Fukushima, Japan). The patients’ hospital records were retrospectively analyzed to assess patient background, perioperative and postoperative outcomes, as well as the tumor clinicopathological characteristics. The following surgical data were included in the analyses:the collection route of the resected tumor; the resection site closure method; the operation time; and the estimated blood loss. The following postoperative data were collected; modified-Fletcher’s classification, tumor size, postoperative complications (bleeding, leakage, delayed gastric emptying, infection including surgical site infection), postoperative hospital stay length, tumor recurrence, and mortality.
The preoperative workup for all patients included reviews of the medical history, standard blood tests, an upper gastrointestinal endoscopy with endoscopic ultrasonography examination, and computed tomography. All patients had previously undergone endoscopic ultrasonography-guided fine-needle aspiration and were histologically diagnosed as having a gastric GIST by immunohistochemistry.
The surgical treatment algorithm for GIST in our hospital is shown in Figure 1. LECS for gastric GIST was not indicated for patients with a previous history of gastrectomy or multiple laparotomies. LECS was indicated for gastric GISTs of ≤ 50 mm in diameter in the preoperative diagnosis and for tumors with intraluminal or intramural growth, excluding those with typical extraluminal growth. Since 2017, all patients with intraluminal or intramural tumors in our hospital have undergone inverted LECS according to our treatment protocol, whereas CLEAN-NET or closed LECS was performed on the tumors with delle, depending on the tumor size.
Patients underwent postoperative CT scans and endoscopies at 6 months, 1 year, and then annually thereafter to diagnose and monitor any local or distant recurrence.

Fig. 1.
Surgical treatment algorithm for gastric GIST in our hospital
The previous endoscopic and laparoscopic approaches in the classical LECS procedure were as follows. The patients are placed in the lithotomy position under general anesthesia and the operating room laid out as shown in Figure 2. A laparoscopic camera port is inserted into the umbilicus with the open technique. Four additional ports (one 12-mm port and three 5-mm ports) are inserted into the upper right, lower right (12-mm port), upper left and lower left quadrants under a 10 mmHg pneumoperitoneum by carbon dioxide gas. The tumor is completely resected endoscopically using an endoscopic submucosal dissection technique and full layer dissection of the gastric wall. The stomach wall defect is sutured using a linear stapler or a laparoscopic hand-sewn technique with a self-anchoring barbed suture. This combined endoscopic and laparoscopic approach enables us to resect the tumor lesion with as narrow a margin as possible. Resected specimens sized ≤ 30 mm in diameter are put in collection bags and retrieved per orally, while specimens sized > 30 mm are retrieved via the laparoscopic camera port site at the umbilicus.
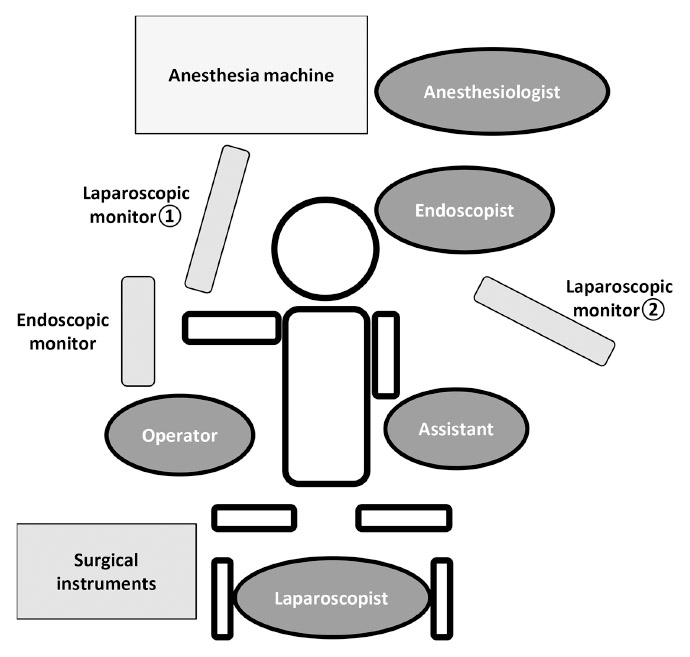
Fig. 2.
Setup for LECS
The routine endoscopic and laparoscopic approaches in the inverted LECS procedure are as follows. After identification of the tumor under laparoscopy and endoscopy, the gastric wall around the tumor is lifted circumferentially by three or four stitches. Each of the stitches is appropriately pulled out of the abdominal port site like a crown4). Then, the tumor is completely resected endoscopically using an endoscopic submucosal dissection technique and full layer dissection of the gastric wall. The stomach wall defect is sutured using a laparoscopic hand-sewn technique with a self-anchoring barbed suture.
Surgical procedures of CLEAN-NET and closed LECS
Inoue et al. and Kikuchi et al. developed novel non-exposure techniques, termed ‘CLEAN-NET’ and ‘closed LECS’, respectively5, 6). CLEAN-NET is a procedure to harvest the tumor into the abdominal cavity, while closed LECS is a procedure to harvest the tumor into the gastric tube cavity. We perform these surgical procedures based on the description in the original articles. Briefly describing CLEAN-NET, the surgery is performed by incising the serosal muscular layer around the tumor in the abdominal cavity without destroying the mucosa. Once the serosa of the tumor is dissected circumferentially, the full layer is lifted, and the mucosa is stretched. Then, the mucosa is cut with a stapling device, and a full-layer resection of the specimen is achieved. Both procedures enable resection without perforation of the stomach lumen and the abdominal cavity. In contrast, in the closed LECS, a circumferential incision is made endoscopically around the tumor using the endoscopic submucosal dissection technique, and then the serosal muscle layer corresponding to the submucosal dissection line is sutured in a straight line laparoscopically. Consequently, the gastric wall containing the tumor protrudes into the gastric cavity. Finally, an endoscopic serosal muscle layer incision is performed along the submucosal dissection line, and the entire gastric wall is dissected. The gastric wall defect caused by tumor resection is endoscopically closed with clips, and the resected lesion is orally retrieved. CLEAN-NET is indicated for tumors of > 30 mm in diameter with delle. On the other hand, closed LECS is indicated for tumors of ≤ 30 mm in diameter with delle, because the resected specimen needs to be retrieved orally.
Ethics Statement
This prospective observational study was approved by the Fukushima Medical University Certified Review Board (no. 3475).
Human rights statement and informed consent
All procedures were performed in accordance with the ethical standards of the relevant committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as well as its later versions. Informed consent or a substitute for it was obtained from all patients regarding their participation in the study.
Results
The clinical backgrounds of the patients are shown in Table 1, and the clinicopathological characteristics of the gastric GISTs are presented in Table 2. Twenty-two of the 40 tumors were located in the upper gastric portion. The average tumor size was 25 ± 9 mm. The operation time for LECS was 166 ± 44 min, and the estimated blood loss was 25 ± 87 ml (Table 3). The resection line around the tumor was cautiously determined by endoscopically and laparoscopically confirming the tumor margin. In all cases, the tumor was not exposed at the resection margin and no additional resection was required. Intraoperative diagnosis of the resection margins is usually not performed. In two cases with delle on the mucosal surface of the tumor, the tumors were resected without damage to the capsule. None of the forty cases were converted to open surgery. Postoperative complications occurred in five cases:one postoperative bleeding, one delayed gastric emptying, and three postoperative infections, of which two were pneumonia (both C-D grade II), and one was a bacteremia of unknown origin (C-D grade II). The patient who developed the postoperative bacteremia had undergone classical LECS. Although no detectable intra-abdominal abscess was identified on CT imaging, organ space surgical site infection could not be ruled out. There was only one case of C-D grade III postoperative complications after closed LECS, in which endoscopic hemostasis with clipping was performed for postoperative hemorrhage. The median postoperative hospital stay was 7 days. One patient was found to have a high risk of recurrence according to the modified-Fletcher’s classification although no adjuvant chemotherapy was administered because of a coexisting cancer of another organ. All patients were followed up for a median period of 31 months (range 1-98 months). No patients showed any local or peritoneal recurrence (Table 4).

Discussion
In the present report, we demonstrate the satisfactory clinical outcomes of LECS for gastric GIST in 40 consecutive cases treated at our institution.
As for postoperative short-term outcomes, it has been previously reported that there were no severe complications after LECS. For example, Hiki et al. reported no complications in seven cases3), and Waseda et al. reported two cases of gastric motility disorder in 22 cases9). In the current study of 40 cases, we also confirmed that the LECS procedure is a safe and feasible technique, although one patient who underwent closed LECS developed postoperative bleeding and required endoscopic hemostasis. In the closed LECS, because the resection site is closed endoscopically with a clip, the suture site may require closer monitoring for troubles such as bleeding or perforation of the suturing site compared to those closed by other laparoscopic suturing techniques. Patients who have undergone closed LECS should be carefully monitored for progression of anemia and abdominal symptoms such as pain and bloody stools.
Regarding oncological outcomes, Tsujimoto et al. reported no local or systemic recurrence in 20 cases with a median follow-up of 20.7 months10). In the current study, we also confirmed that there were no recurrent cases within the median follow-up period of 31 months. Technically, LECS excluding CLEAN-NET and closed LECS involves intentional perforation of the gastric lumen to the abdominal cavity. Therefore, there may be a risk of tumor seeding; however, none of the 40 patients in the present study developed recurrence associated with dissemination. Considering that GIST is less invasive in comparison to other tumors, the LECS technique is a useful and acceptable curative surgical procedure.
It is generally accepted that the advantages of LECS for GIST over laparoscopic wedge resection are that it minimizes the resection area with a sufficient safety margin, and it prevents stomach deformity. Kawahira et al. reported that in 16 cases treated by LECS the size of the surgical specimen was sufficiently and radically minimized compared with nine cases treated by laparoscopic wedge resection7). Namikawa et al. reported that an important advantage of LECS was the reduction in the resected area of the gastric wall compared to that in laparoscopic wedge resection8). In the current study, with a relatively larger case series, we confirmed the clinical effectiveness of LECS for intraluminal and intramural type GIST. Considering a balance between surgical invasiveness and curability, we believe that LECS for intraluminal and intramural type GIST is the ideal treatment option.
There are several limitations to this study. First, there were several different surgeons and assistants within the observation period of this study, which may have affected surgical outcomes; for example, one patient early after the introduction of LECS had excessive intraoperative blood loss (535 ml), which was from the stomach wall at the tumor resection site. The tumor was located in the anterior wall of the gastric antrum, and the thick gastric wall with rich intramural vessels resulted in a high amount of bleeding. Depending on the location of the tumor, LECS should be performed by a surgeon with considerable surgical experience. Second, the results of the present study may be insufficient to describe postoperative or oncological safety because the patient population is not large enough in number and includes some cases with short observation periods. Further observation is necessary to accumulate more cases for analysis.
In conclusion, LECS is a safe and useful surgical procedure with good clinical outcomes for gastric intraluminal and intramural type GIST ≤ 50 mm in diameter.
Conflict of interest disclosure
The Authors declare no conflict of interests for this article.
References
- 1. Koo DH, Ryu MH, Kim KM, et al. Asian consensus guidelines for the diagnosis and management of gastrointestinal stromal tumor. Cancer Res Treat, 48(4):1155-1166, 2016.
- 2. Japan Society of Clinical Oncology. “Clinical Practice Guideline, GIST:gastrointestinal stromal tumor”. http://www.jsco-cpg.jp/item/03/index.html.
- 3. Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc, 22(7):1729-1735, 2008.
- 4. Nunobe S, Hiki N, Gotoda T, et al. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer, 15(3):338-342, 2012.
- 5. Inoue H, Ikeda H, Hosoya T, et al. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond:full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am, 21(1):129-140, 2012.
- 6. Kikuchi S, Nishizaki M, Kuroda S, et al. Nonexposure laparoscopic and endoscopic cooperative surgery (closed laparoscopic and endoscopic cooperative surgery) for gastric submucosal tumor. Gastric Cancer, 20(3):553-557, 2017.
- 7. Kawahira H, Hayashi H, Natsume T, et al. Surgical advantages of gastric SMTs by laparoscopy and endoscopy cooperative surgery. Hepatogastroenterology, 59(114):415-417, 2012.
- 8. Namikawa T, Hanazaki K. Laparoscopic endoscopic cooperative surgery as a minimally invasive treatment for gastric submucosal tumor. World J Gastrointest Endosc, 7(14):1150-1156, 2015.
- 9. Waseda Y, Doyama H, Inaki N, et al. Does laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors preserve residual gastric motility? Results of a retrospective single-center study. PLoS One, 26; 9(6):e101337, 2014.
- 10. Tsujimoto H, Yaguchi Y, Kumano I, Takahata R, Ono S, Hase K. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J Surg, 36(2):327-330, 2012.






