2022 Volume 68 Issue 3 Pages 175-178
2022 Volume 68 Issue 3 Pages 175-178
Ravulizumab is an anti-C5 antibody approved for treating paroxysmal nocturnal hemoglobinuria (PNH). In August 2019, a 77-year-old Japanese man with PNH, who had been on ravulizumab treatment for 2 years, was hospitalized for chest discomfort and malaise. Electrocardiography identified a right bundle block, and elevated serum troponin I and d-dimer suggested ischemic heart disease. Cardiac catheterization revealed severe stenosis in the left anterior descending coronary artery, and intracoronary stenting relieved his chest discomfort. The final diagnosis was unstable angina unrelated to ravulizumab, and the patient’s ravulizumab treatment was uninterrupted with no significant complications of PNH. This case report highlights the importance of continuing complement inhibition therapy during acute coronary events.
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, non-neoplastic, acquired hematopoietic stem cell disorder1). Eculizumab, a human monoclonal antibody that blocks complement protein C5, is now considered the standard of care for treating PNH2,3). Although it is highly effective, approximately 11%-27% of patients who receive approved dosages of eculizumab for PNH experience potentially life-threatening breakthrough hemolysis (BTH)4-6). BTH is defined as at least one new or worsening symptom or sign of intravascular hemolysis (fatigue, hemoglobinuria, abdominal pain, dyspnea, anemia [hemoglobin <10 g/dL], major adverse vascular event [including thrombosis], dysphagia, or erectile dysfunction) in the presence of elevated lactate dehydrogenase (LDH) ≥2× the upper limit of normal (ULN) after prior reduction of LDH to <1.5×ULN on treatment7,8). Causes of BTH include suboptimal inhibition of C5 and/or complement-amplifying conditions that arise due to infection, surgery, or pregnancy9,10).
Ravulizumab is a newly developed, human anti-C5 antibody derived from eculizumab, with an 8-week dosing interval11). Ravulizumab demonstrated noninferiority to eculizumab for the endpoints, including LDH normalization and incidence of BTH, in two international phase 3 trials (including Japan); one in complement inhibitor-naïve patients (study 301 [NCT02946463])7) and the other in eculizumab-experienced patients (study 302 [NCT03056040])8). In study 301, five non-Japanese patients experienced BTH while receiving ravulizumab despite complete blockade of free C5 (free C5 <0.5 µg/mL) and 13 experienced BTH during treatment with eculizumab7,12). By comparison, in study 302, none of the patients who were stable on eculizumab and then switched to ravulizumab developed BTH while receiving ravulizumab and none experienced thromboembolic events;but five non-Japanese patients who received eculizumab experienced seven BTH events8,12).
Situations like infection, pregnancy, or surgery could increase complement activation placing patients on eculizumab or ravulizumab at risk of BTH. Here, we describe our experience of a 77-year-old male Japanese patient with PNH who was successfully continued on ravulizumab during an acute event of unstable angina (non-ST-elevation myocardial infarction).
The patient was initially diagnosed with PNH in June 2016 after visiting a local hospital complaining of general malaise. Blood tests performed at the time indicated hemolytic anemia with values for hemoglobin of 7.4 g/dL, platelet count of 18.4 × 104/mL, total bilirubin of 1.6 mg/dL, and LDH of 1,208 IU/L (5.4×ULN). Flow cytometry analysis found that the percentage of CD55-deficient red blood cells was 56.7% and CD59-deficient red blood cells was 42.7%, consistent with a diagnosis of PNH.
The patient was transferred to our hospital, enrolled in study 301 and randomized to eculizumab, which was initiated after meningococcal vaccination (~8 months after initial presentation at the local hospital). The patient’s LDH value promptly decreased to an almost normal level and he continued to make good clinical progress for the next 6 months. The patient did not experience any BTH events during the randomized treatment period. After the 6-month randomized period, the patient was safely transitioned to ravulizumab in the study’s extension phase.
In August 2019, the patient was admitted to our hospital complaining of chest discomfort, 6 weeks and 4 days after his most recent dose of ravulizumab. He initially presented with a hemoglobin level of 13.2 g/dL and elevated levels of LDH (373 IU/L [1.7×ULN]) and bilirubin (8.1 mg/dL) (Figure 1). The next day, his LDH had increased to 514 IU/L [2.1×ULN] and he reported symptoms of fatigue and shortness of breath, both of which are related to PNH. His body temperature was 37.3°C, C-reactive protein level was 9.61 mg/dL, and white blood cell count was 10,000/mL, suggesting a possible infection as the cause of BTH. The patient’s blood culture was negative for bacteria. Blood tests conducted as part of the extension of study 301 showed that the serum ravulizumab level was 425 µg/mL and free C5 was appropriately low at 0.0626 µg/mL. There were no additional data to support a complement-amplifying condition.
Echocardiography indicated left ventricular (LV) wall motion with an LV ejection fraction of 63%, asynergy, and hypokinesis. Dilatation of the left atrium was found, but not LV dilatation. There was thickening/calcification of three atrioventricular leaflets. No right-sided enlargement was observed. The diameter of the inferior vena cava was 9 mm. Ischemic heart disease was suspected based on the echocardiography findings. Electrocardiogram showed a right bundle block and serum levels of troponin I and d-dimer were elevated, suggesting ischemic heart disease. Cardiac catheterization found severe stenosis in the left anterior descending coronary artery, leading to an initial diagnosis of acute myocardial infarction. The stenosis was determined to be arteriosclerotic. Intravascular ultrasound was not performed. Because ST depression was found, but there was no evidence of ST elevation, he was diagnosed with non-ST-elevation myocardial infarction. Intracoronary stenting with a drug-eluting stent (XIENCE Sierra;diameter:2.75 mm;length: 15 mm) was performed. The patient took aspirin (100 mg once-daily for 8 months) and is still taking clopidogrel (75 mg once-daily). Ceftriaxone was commenced empirically and ravulizumab therapy was uninterrupted. Levels of bilirubin, C-reactive protein, and LDH decreased. The final diagnosis was unstable angina related to age and history of hypertension that was unrelated to ravulizumab treatment. The last dose of ravulizumab in the extension phase was 2 years and 13 days after enrolment in the study. The patient continues to receive commercially available ravulizumab.
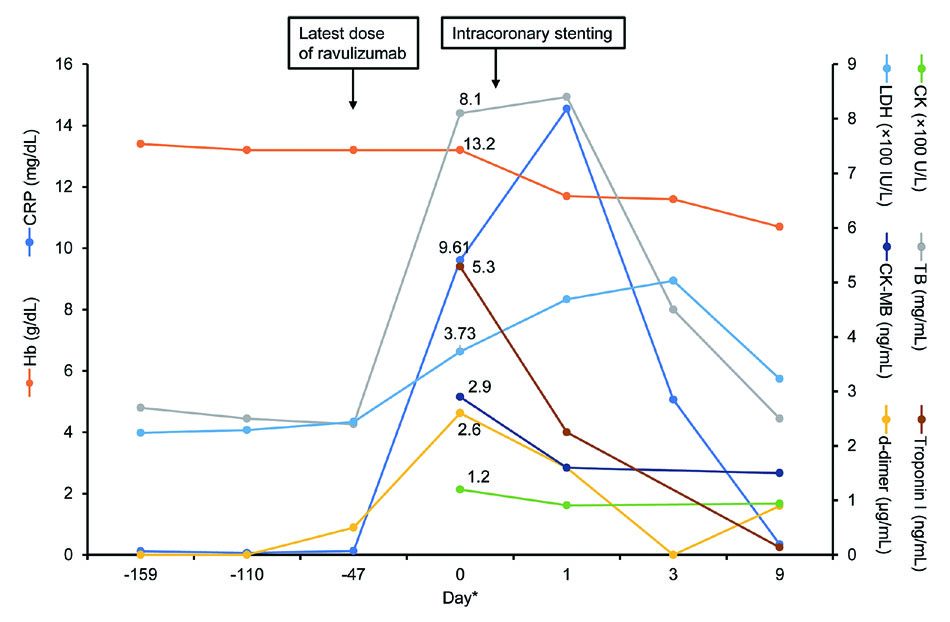
*Day 0:presentation in August 2019;days −159, −110, and −47:previous doses of ravulizumab
CK, CK-MB, and troponin I levels measured in February 2017 were 97 U/L, 1.5 ng/mL, and 0.032 ng/mL, respectively. The corresponding values in October 2019 were 84 U/L, 1.2 ng/mL, and 0.017 ng/mL, respectively.
CK, creatine kinase;CK-MB, creatine kinase myocardial band;CRP, C-reactive protein;Hb, hemoglobin;LDH, lactate dehydrogenase;TB, total bilirubin
We have described a 77-year-old Japanese patient who experienced a coronary event while receiving ravulizumab. The LDH and symptoms met the protocol-specified definition of BTH but could also be explained by the acute cardiac event. Further investigations revealed complete terminal complement blockade. At the clinical assessment, his serum levels of ravulizumab approached the trough levels observed in studies 301 and 302 (data not shown), and his free C5 levels remained less than 0.5 µg/mL, suggesting complete inhibition of terminal complement. Intracoronary stenting relieved the patient’s cardiac symptoms. Levels of bilirubin, C-reactive protein, and LDH also decreased. LDH is known to be found in cardiac muscle as well as in red blood cells. The patient received one dose of ravulizumab during the extension phase and is receiving commercially available ravulizumab with no interruptions of therapy.
In this case report of a patient with unstable angina treated successfully with no PNH-related complications, we highlight the importance of continuing complement inhibition therapy during acute coronary situations such as myocardial infarction to prevent BTH and possible subsequent thrombosis. Data from studies 301 and 302 show that the rate of BTH was low in patients who received ravulizumab. The long half-life of ravulizumab reduces the treatment burden of patients with PNH and decreases the risk of developing BTH due to suboptimal C5 inhibition. The clinical and laboratory findings highlight the importance of continuing administration of a terminal complement inhibitor, such as ravulizumab, during acute coronary events.
Editorial support was provided by Apurva Davé, PhD, of Medical Expressions (Chicago, IL, USA) and Nicholas D. Smith, PhD (EMC Japan K.K., Japan). Publication of this case report was funded by Alexion Pharma Inc.
T.Sa., K.H., S.K., H.O., and T.I. were investigators in ALXN1210-PNH-301 and 302 studies. The manuscript was reviewed by Alexion Pharma Inc. and Alexion Pharma G.K. Alexion, the sponsor of the study, contributed to scientific accuracy review of the data. Changes resulting from comments received were made by the authors on the basis of scientific and editorial merit.
The patient was a participant in study 301 (approved by the local IRB). The patient consented to the use of his medical data.