1. Introduction
Coumarin is a naturally occurring organic chemical that is often ingested as part of
cinnamon-containing foods. Although the intake of coumarin from foods is generally
considered to be safe, coumarin-induced hepatotoxicity has been reported to occasionally
occur in humans1,2). Other than through the food intake,
a clinical trial of coumarin has been performed on lymphedema patients, but the occurrence
of hepatic disorders led to its withdrawal3,4,5). The US Food and Drug Administration (FDA) has banned the use
of coumarin as a food additive due to hepatotoxicity concerns.
The occurrence of hepatotoxicity has been observed in experimental animals such as dogs and
rats after the administration of coumarin. The coumarin-induced hepatotoxicity is believed
to be associated with the metabolism in the body. Coumarin is metabolized to
o-hydroxyphenylacetic acid (o-HPA) through the reactive
metabolite coumarin 3,4-epoxide6,7),
while the biological 7-hydroxylation is considered to be a process of detoxification.
Species differences clearly exist between humans and rats in terms of the coumarin
7-hyroxylation. In fact, the detoxification rates are rapid in humans and slow in
rats8,9,10,11). Only CYP2A6 catalyzes coumarin 7-hydroxylation among the
major cytochrome P450 enzymes in the human liver. This major detoxification pathway mediated
by CYP2A6 is susceptible to genetic polymorphisms, but the influence of such genetic
polymorphisms on the hepatotoxicity of coumarin still remains unclarified in
vivo in humans12).
Moreover, multiple CYP enzymes participate in the 3,4-epoxidation, and the hepatic levels of
these enzymes are known to vary among individuals. Therefore, an understanding of inter- and
intra-individual differences in terms of their coumarin intake amounts and of their
metabolic capacities is necessary in order to evaluate the risk of food-derived coumarin in
humans.
In this study, human-relevant data on coumarin were collected, including data regarding its
intakes from food, absorption, distribution, metabolism, and excretion (ADME), toxicity, and
clinical information in an attempt to refine its risk assessment. Furthermore, two
in silico models were introduced in order to provide additional
human-relevant information: a physiologically-based pharmacokinetic (PBPK) model11,13), and a drug-induced liver injury (DILI)
severity-predicting model developed by the FDA (hereafter referred to as the FDA DILI score
model)14).
2. Materials and Methods
2.1 Intake, ADME and Toxicity Data Collection
Data on coumarin (including intake from food, experimental and clinical studies on the
ADME and the in vivo and in vitro toxicity of coumarin)
were obtained from the relevant regulatory assessment reports1,15,16,17,18,19) and the literature.
2.2 PBPK Modeling
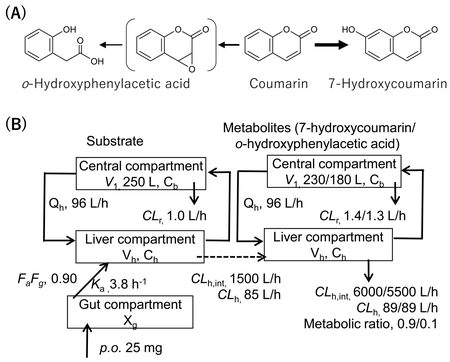
The human PBPK models were constructed based on the rat PBPK model consisting of the gut,
the liver, and the central compartments, as described previously11). The blood concentration profiles in rats
treated orally with 200 mg/kg of coumarin were reproduced11). A scale-up strategy was applied by using the
fixed values of the human body weight (70 kg) and the liver volume (1.5 L). The input
parameters for the human PBPK model are presented in detail in Fig. 1. Systems of differential equations were used in order to
obtain the concentrations of the substrate coumarin as well as those of the two
metabolites (7-hydroxycoumarin and o-HPA) in the central compartments, as
described previously11).
2.3 Application of the FDA DILI Score Model for Coumarin and Related Drugs
Coumarin and related drugs sharing a substructure with coumarin (e.g., warfarin and
methoxsalen) were applied to the FDA DILI score model14). The DILI scores were calculated by using the following
formula: DILI score = 0.608 × loge (daily dose in mg) + 0.227 × logP + 2.833
(in the case of a present reactive metabolite formation). Prior to the application, the
logP (the ratio of the concentration of the unionized compound at equilibrium between
organic and aqueous phases) and the molecular weight (MW) were calculated by using the
ADMET Predictor version 10.0 (SimulationsPlus, USA). Furthermore, the chemical classes of
every substructure of coumarin were compared with those of the set molecules used for the
construction of the model (a total of 354 human pharmaceuticals). The chemical classes of
the substructures were searched in the public ChemoTyper application (Altamira LLC, USA
and Molecular Networks GmbH, Germany). The chemical structures were profiled with the use
of the OECD QSAR Toolbox20)
(ver. 4.4) for both coumarin and the set molecules.
3. Results
3.1 Collected data of Intake of Coumarin from Foods
In the European Food Safety Authority (EFSA) opinion of 2004, a theoretically-calculated
maximum daily intake of coumarin was estimated to be 1.5 mg for an adult with a default
body weight of 60 kg (0.025 mg/kg of body weight per day)15). A mean coumarin intake from foods consumed
within the Christmas season was estimated to be 5.0 mg per week, and the intakes of the
heaviest consumers (six subjects) exceeded 35 mg/week2). In Japan, a
consumption of 2.45 g of cinnamon corresponding to the ingestion of 2.73 mg of coumarin
from daily foods has been reported21). The total amount of coumarin consumed can reach 5.18
mg/day if daily supplements that contain cinnamon are also consumed21). In the worst-case scenarios, a
food-mediated coumarin consumption of around 60 mg/day (based on a body weight of 50 kg)
has been reported in Norway18), and an additional exposure of 18 mg/day coumarin was
calculated to occur from food supplements in Germany16). These results suggest an average intake of approximately
1−3 mg of coumarin per day, as well as a level equal to ten-times higher the average
intake in the worst-case scenario.
3.2 Collected data of Absorption, Distribution, Metabolism, and Excretion of
Coumarin
In an in vitro intestinal epithelial cell monolayer system, a
fraction-absorbed value of >0.9 was estimated for coumarin based on its apparent
permeability. Coumarin is rapidly absorbed after an oral intake of 0.857 mg/kg, but its
availability to the systemic circulation is reported to be less than 4%13). The rest of the intake appeared
in the form of 7-hydroxycoumarin and the glucuronide in the systemic-circulation, thereby
suggesting an extensive first-pass effect. In a study employing an oral administration of
200 mg of coumarin in seven different subjects, 63.4% of the dose was recovered in the
24-h urine in the form of total 7-hydroxycoumarin22). Coumarin can also be absorbed fairly efficiently after a
dermal application, and the absorption rates ranged from 54.7% to 66.1% in
humans10).
The in vivo metabolic profiles of coumarin are similar among
experimental animals and humans. Metabolites deriving from the oxidation of both the
phenyl ring (7-hydroxylation) and the lactone ring (3,4-oxidation) can be detected mostly
in the urine of mice, rats, dogs10), and human volunteers23); however, certain extents of a fecal excretion have also
been observed in rats exposed to high doses10).
7-Hydroxycoumarin and its glucuronic acid conjugate were the major metabolites detected
in the urine of most individuals, while the lactone-ring opening metabolite,
o-HPA, was slightly detected in urine; interestingly, the amount of
o-HPA was more than that of 7-hydroxycoumarin in the 8-h urine of some
individuals after a 2-mg coumarin intake23). o-Hydroxyphenylacetaldehyde can be formed
in vitro by microsomes from all four human liver samples as the major
metabolite of coumarin at a coumarin concentration of 1 mM; however, 7-hydroxycoumarin was
the major metabolite detected after an exposure to coumarin concentrations below 50
μM24). These results
suggest that both the coumarin concentration and the genetic background can affect
coumarin metabolism in humans.
Studies of the toxicity mechanism of coumarin have consistently indicated a role for the
reactive intermediate in coumarin-induced hepatotoxicity. The microsomal formation of
metabolites bound covalently to hepatic proteins8), the identification of
o-hydroxyphenylacetaldehyde as a major metabolite of coumarin in the rat
hepatic microsomes8), the much
lower toxicities of 3- or 4-methylcoumarin and of 3,4-dimethylcoumarin than
coumarin25), as well as
the reactivity of o-hydroxyphenylacetaldehyde26); all support the production of a reactive
3,4-oxide for the facilitation of the coumarin-mediated hepatotoxicity.
In human recombinant CYP systems, CYP1A1, CYP1A2 and CYP2E1 mediate the formation of
o-hydroxyphenylacetaldehyde, and CYP3A4 may also support this
reaction27,28). The 7-hydroxycoumarin formation
is supported only by CYP2A6, and no activities are detected with CYP1A1, CYP1A2, CYP2E1,
and CYP3A428). CYP2A13
catalyzes both the 3,4-oxide formation and 7-hydroxylation of coumarin29). The low levels of CYP2A13 are
expressed selectively in extra-hepatic tissues, while the enzyme’s levels in the liver are
negligible30). Population
studies suggest that 6% of the UK population is homozygous for the mutant CYP2A6 alleles,
whereas the mutant CYP2A6 allele frequency may be as high as 48% in Japanese
subjects31).
The production of o-HPA was correlated with the CYP1A2 content of human
hepatocytes32). The
production of o-HPA in the human liver microsomal system as well as
in vivo, in the humanized-liver mice, was clearly inhibited in the
presence of a selective inhibitor of CYP1A2, furafylline33,34). These results suggest, at least partly, the involvement of
CYP1A2 in the metabolic activation of coumarin in the human liver. CYP2E1 has also been
expected to mediate the 3,4-epoxidation of coumarin in humans27,35,36).
3.3 Collected data of Toxicity of Coumarin
Toxicity data of coumarin have been evaluated and published in the form of review
articles2,10,37,38) and risk assessment reports1,15,16,17,18,19). These data consistently indicate the liver as the most
sensitive target of coumarin in experimental animals. Hepatotoxicity in rats includes
hepatic histopathological lesions along with increased liver enzymes at doses ≥50
mg/kg/day in a 2-year-long carcinogenicity study19). Slight jaundice, marked histopathologic hepatic changes,
and distended gall bladder were observed in a 1-year chronic study in dogs39). In baboons, hepatic changes
were limited to an increased liver weight and an hepatocyte endoplasmic reticulum
hypertrophy observed at the highest coumarin dose (67.5 mg/kg/day) in a 2-year-long
chronic toxicity study40).
The EFSA determined the tolerable daily intake (TDI) of coumarin to be 0.1 mg/kg/day,
based on the no-observed-adverse-effect level (NOAEL) of 10 mg/kg/day found in a chronic
toxicity study of coumarin in dogs, with an uncertainty factor of 10015).
Hepatic disorder, characterized as an elevation of the liver enzyme levels, is the most
common coumarin-associated adverse finding reported in humans1,2). In one case, a 23-year-old woman was hospitalized with
hepatitis after consuming 1–2 g of cinnamon (equivalent to 3.3–6.6 mg of coumarin) daily,
for two months2). According to
the expert report on the assessment of coumarin in medicinal products1,2), liver damage cannot be ruled out at a daily dose of 25 mg
coumarin for a part of the population. In order to extrapolate from this effect level to a
human NOAEL, a factor of 5 is considered as justified in the case of a severe effect at
the lowest observed adverse effect level. Thus, an exposure to 5 mg of coumarin per day is
expected to cause no adverse effects in sensitive subjects. Moreover, a TDI of 0.1 mg/kg
body weight was derived1,2,16). This value agreed well with the EFSA value based on animal
data15).
The incidence of coumarin-induced hepatotoxicity was estimated to be 0.37% by a clinical
trial41). In the National
Institutes of Health LiverTox Database, the idiosyncratic, clinically apparent liver
injury associated with coumarin was estimated to occur in 2 out of 1,000 patient-years of
use42).
3.4 Estimation of the Internal Exposure to Coumarin in Humans Using the PBPK
Model
Pharmacokinetic data of coumarin in humans are necessary in order to assess the body
exposure in vivo and the subsequent hepatotoxicity. However, the
available data that are relevant to the assessment of possible toxic dose levels are
limited43). Therefore,
the plasma concentrations of coumarin, 7-hydroxycoumarin, and o-HPA
(generated via a coumarin 3,4-epoxidation) were estimated by using human
PBPK models that have been previously developed and validated11). Virtual oral doses of 2.5 mg and 25 mg were
applied in the present study. The former dose corresponds approximately to the average
intake of food-derived coumarin21), and the latter dose corresponds to an amount close to the
maximum estimate of coumarin intake from food or the amount of pharmaceutical
administration that cannot exclude hepatotoxicity in a part of the human
population2). Plasma
concentration curves after a single or a 28-day repeated virtual administration of
coumarin in humans were generated by using a human PBPK model (Fig. 2). After a single dosing, the mean and the maximum plasma
concentration after an intake of coumarin at a dose of 2.5 mg/day was estimated to be 0.04
and 0.29 ng/mL (0.27 and 2.0 nM), respectively. The results for the 25-mg oral dose are
presented in Fig. 2A. The mean and the maximum
plasma concentrations of coumarin after a single administration were 0.41 and 2.9 ng/mL
(2.8 and 20 nM), respectively. The plasma concentration curves after the repeated virtual
administration of coumarin in humans for 28 days were also generated by using the human
PBPK model (Fig. 2B). The respective
concentrations of coumarin, 7-hydroxycoumarin, and o-HPA after a repeated
dosing were comparable to those after a single dosing, in consistent with the notion that
coumarin is rapidly cleared and does not accumulate in the body.

The possible hepatotoxicity of coumarin was evaluated by using a FDA DILI score model
based on the daily dose of the substance, lipophilicity, and reactive metabolite
formation14).
The applicability of coumarin to the FDA DILI score model was assessed by plotting the
logP and the MW of coumarin and the set molecules (Fig.
3). The estimated logP value of coumarin (1.39) was in the range of most of the
set molecules (from −4 to 8), and the inclusion of the MW of coumarin (146.14) was also
confirmed. Coumarin consisted of a total of 13 chemotypes (including benzopyrone), all of
which were included in a set of chemotypes of the set molecules (Table 1 and Supplementary Data Table S1). The
chemical structure profiling that was undertaken by using the OECD QSAR Toolbox yielded
similar results (Supplementary Data Table S2).

Table 1.
Chemotype of coumarin and duplication in the set of 354 molecules for
constructing the FDA DILI score model
| Chemotype contained in coumarin |
No. of duplications in the set molecules |
| bond:C(=O)O_carboxylicEster_alkenyl |
9 |
| bond:C=O_carbonyl_generic |
228 |
| chain:alkeneCyclic_ethene_C_(connect_noZ) |
37 |
| chain:alkeneCyclic_ethene_generic |
67 |
| chain:aromaticAlkane_Ph-C1_cyclic |
83 |
| chain:aromaticAlkene_Ph-C2_cyclic |
11 |
| ring:aromatic_benzene |
264 |
| ring:hetero_[6]_O_pyran_generic |
15 |
| ring:hetero_[6]_Z_1- |
100 |
| ring:hetero_[6]_Z_generic |
152 |
| ring:hetero_[6_6]_O_benzopyran |
3 |
| ring:hetero_[6_6]_O_benzopyrone_(1_2-) |
1 |
| ring:hetero_[6_6]_Z_generic |
52 |
Coumarin and the related drugs sharing a substructure with coumarin were applied to the
FDA DILI score model (Table 2). The
epoxidation of coumarin and of methoxsalen were assumed to lead to the formation of
reactive intermediates15,16). The DILI scores of coumarin were calculated to be 3.71 and
5.11 for the daily consumption levels of 2.5 and 25 mg/day, respectively, and the risk was
judged to be moderate (score of 3–6). Warfarin (10 mg/day) was judged to be of low risk
based on a DILI score of 1.95, whereas methoxsalen (3 mg/day) was considered to be of
medium risk based on a DILI score of 3.92.
Table 2.
Application of coumarin and related drugs to the FDA DILI score model
| Chemicals |
Daily Dose
(mg/day) |
logP |
RM
formation |
DILI
score |
DILI
risk |
Remarks |
Coumarin
 |
2.5 |
1.39 |
Yes (1) |
3.71 |
M |
Daily dose as food with very occasional elevated liver
enzymes |
| 25 |
5.11 |
M |
Daily dose as medicine for lymphedema and occasionally
taken as food with occasional elevated liver enzymes |
Warfarin
 |
2 |
2.44 |
No (0) |
0.98 |
L |
Daily dose as medicine (anticoagulant)
with rare hepatotoxicity cases |
| 10 |
1.95 |
L |
Methoxsalen
 |
3 |
1.93 |
Yes (1) |
3.92 |
M |
Daily dose as medicine for psoriasis with
occasional elevated liver enzymes (2%–12% of patients) |
| 40 |
5.51 |
M |
DILI score based on daily dose, lipophilicity (logP), and presence (1) or absence (0)
of reactive metabolite (RM) formation was calculated following the formula: DILI score
= 0.608 × loge(daily dose by mg) + 0.227 × logP + 2.833 × 1/0. DILI risk
was classified according to DILI score as low (L, <3), moderate (M, 3–6), and high
(H, >6)
4. Discussion
In this study, the hepatotoxicity of food-derived coumarin in humans was comprehensively
reevaluated by the use of the data of existing clinical and animal studies as well as those
of generated by us through in silico approaches for humans.
According to several studies, the average intake of food-derived coumarin is about 1−3
mg/day, and in the worst-case scenarios, the intake is expected to be about ten-times higher
than that of the average amount. The levels were found to be almost equal to or above the
TDI (0.1 mg/kg/day) that derived from animal studies15).
It has been shown that the majority of coumarin is rapidly converted to 7-hydroxycoumarin,
along with low levels of o-HPA which is associated with the metabolic
activation (Fig. 2A). At a virtual intake of 25 mg
of coumarin (that is approximately ten-times more than the average intake), the maximum
plasma concentrations of coumarin and o-HPA were expected to be 20 nM and 2
nM, respectively, at 0.7 h (Fig. 2A). Based on the
plasma-to-liver distribution ratios of coumarin and o-HPA of 0.875 and
0.50411), the coumarin and
o-HPA concentrations in the liver at the same timepoint were estimated to
be 17 nM and 1.1 nM, respectively.
The maximum blood concentrations of coumarin and o-HPA were estimated to
be approximately 200 μM and 80 μM, respectively, at 0.5 h after the administration of a
toxic dose of 200 mg/kg44).
Therefore, clear differences were observed with regard to both the coumarin and the
o-HPA levels between the simulated data (at 25 mg/kg) in humans and
measured data (at 200 mg/kg) in rats. Based on the pharmacokinetics data, an intake of 25
mg/kg of coumarin in humans may be considered to be of low or subtle concern for the
development of hepatotoxicity, as far as the human population maintains average levels of
capacity for coumarin metabolism. It should be noted, however, that this PBPK model is based
on data deriving from a small number of healthy individuals. The report by Abraham et al. in
20102) claims that
hepatotoxicity concerns cannot be ruled out in humans after the ingestion of 25 mg or more
of coumarin. These results suggest the existence of a subpopulation that is highly
susceptible to coumarin-induced hepatotoxicity. Further investigation of such individual
differences in terms of the susceptibility to coumarin is warranted.
The detoxification of coumarin in humans is mainly mediated by CYP2A632,35,45), which may imply variations in the susceptibility to coumarin
toxicity as a result of genetic polymorphisms in the metabolizing enzyme46,47,48). The polymorphism of CYP2A6 is rather prevalent in the
Japanese population, and the non-wild (poor metabolizer) types were reportedly present in
nearly half of the population. In addition, both CYP1A2 and CYP2E1 mediate the production of
o-hydroxyphenylacetaldehyde, which is probably associated with coumarin
toxicity. The hepatic levels of CYP1A2 and CYP2E1 vary under the influence of various
environmental factors. Cigarette smoking and alcohol consumption are known to alter the
hepatic levels of CYP1A1/2 and CYP2E1 through induction phenomena, respectively. Other
dietary components are also known to modulate the activation and the detoxification of
coumarin through the processes of inhibition and transport. Therefore, further studies on
the impact of individual differences are required in order to refine the evaluation of the
coumarin-induced hepatotoxicity in humans.
The FDA continues to compile human hepatotoxicity data of approved or withdrawn drugs, and
these data include their daily dose, their lipophilicity, and their reactive metabolite
formation. These data are also used for the construction of the QSAR model aiming to predict
the severity of clinical liver injury14). Available toxicity data of foods and food ingredients are
often not sufficient for the rigid evaluation of their toxicities in humans and, thus, the
use of this model is expected to be beneficial. The applicability was at first checked by
the comparison of the logP, the MW, and the chemotypes of coumarin with those of a total of
354 molecules that were used in order to construct the model. Coumarin was judged to be
applicable, and the daily intakes of coumarin at 2.5 and 25 mg/kg were predicted to be of
moderate risk. The structurally related drug, warfarin, was predicted to be of low risk,
whereas methoxsalen was predicted to be of moderate risk. The estimated results of the
coumarin-related drugs are consistent with hepatotoxicity in humans, thereby offering
information on a relative probability for the development of coumarin-induced
hepatotoxicity. The DILI score model may also be applicable to food ingredients other than
coumarin for the preliminary discrimination or evaluation of potential hepatotoxicity in
humans.
In summary, the possibility of developing coumarin-induced hepatotoxicity in humans was,
herein, reevaluated through a combined approach that integrated the existing data of
clinical and animal studies with data deriving from in silico models. At
the current average coumarin intake, the humans can be considered to be safe, at least as
far as the coumarin-induced hepatotoxicity is concerned. On the other hand, the existence of
a human subpopulation that is highly susceptible to the hepatotoxicity of coumarin is
suggested. Further studies are required in order to achieve a more precise risk assessment
that would take into account the individual differences in coumarin metabolism as defined by
genetic and environmental factors. Moreover, the present study highlights the usefulness of
in silico approaches of pharmacokinetics and the liver injury score model
as battery components of a risk assessment.