2014 Volume 20 Issue 2 Pages 415-421
2014 Volume 20 Issue 2 Pages 415-421
d-Psicose is expected to be widely used as a raw material for various foods in the future due to its beneficial health properties and rheological characteristics. To provide the functional effects of d-psicose to consumers, this sugar needs to be stable during food processing. To evaluate the stability of d-psicose, we investigated changes in d-psicose concentration during caramelization and the Maillard reaction, both of which are important reactions that typically occur during food processing; and during general food processing. During caramelization and the Maillard reaction, the concentration of d-psicose is decreased as the reaction temperature and pH are increased. During food processing, a similar tendency was observed: d-psicose degradation ranged from 3.3% (fig jam) to 10.8% (sponge cake). We concluded that although d-psicose degradation occurs at elevated temperatures and pHs, d-psicose loss can be minimized by controlling the temperature and pH during the cooking process.
d-Psicose (d-ribo-2-hexulose) is a ketohexose and C-3 epimer of d-fructose. Although trace amounts of d-psicose are found naturally in food products and plants (Binkley, 1963; Hough and Stacey, 1966; Luger and Steinhart, 1995; Miller and Swain, 1960; Oshima et al., 2006), this sugar is generally produced commercially using chemical or enzymatic processes (Takamine et al., 2010; Takeshita et al., 2000). Testing of the sugar for safety has verified that d-psicose does not cause mutagenesis or acute toxicity (Matsuo et al., 2002), and the maximum level of d-psicose that does not cause diarrhea in human subjects has been estimated as 0.55 g per kg body weight (Iida et al., 2007). The ability to produce large amounts of d-psicose has enabled various studies to be conducted regarding its use as a foodstuff. d-Psicose has 70% of the sweetness of sucrose and a similar hydration behavior to that of fructose (Ikeda et al., 2011), and proteins that have been glycated with the sugar can be used to produce foods with excellent antioxidant activity and good rheological properties (Puangmanee et al., 2008; Sun et al., 2006). Moreover, d-psicose suppresses blood glucose elevation post-consumption (Hayashi et al., 2010; Iida et al., 2008) and reduces body fat accumulation (Matsuo et al., 2001). This sugar is minimally converted to energy in humans (Iida et al., 2010). Hence, the addition of d-psicose as a substitute for alimentary sugars in foodstuffs might be a feasible strategy for developing new functional foods.
However, reducing sugars, such as ketohexoses, have a tendency to be easily degraded during caramelization and the Maillard reaction under a variety of conditions that are typically encountered during food processing (Wong, 1989). Ajandouz et al. (2001) reported fructose loss at 100°C and high pH in fructose and fructose-lysine aqueous model systems. Many reports have examined the thermal stability of tagatose, which is a C-4 epimer of d-fructose and is known to behave as a prebiotic. Tagatose degradation occurred in buffer solutions that were maintained at 20 – 40°C for 9 months (Dobbs and Bell, 2010) and at higher temperatures associated with thermal processing (Luecke and Bell, 2010), with the greatest extent of degradation occurred in phosphate buffer at higher pH (Dobbs and Bell, 2010; Luecke and Bell, 2010). In conventional beverages, tagatose was not degraded in lemonade at temperatures equal to or less than 61°C, whereas degradation occurred faster in milk because the pH of milk is higher than that of lemonade and the proteins in dairy products enable the Maillard reaction (Bell and Luecke, 2011). d-Psicose has various functions similar to those of tagatose; therefore, its utilization is desirable for the preparation of foodstuffs. However, no reports have been found concerning the stability of d-psicose during food processing.
To evaluate the stability of d-psicose in processed food, we investigated changes in the concentration of d-psicose during general food processing, as well as during caramelization and the Maillard reaction, both of which are important reactions that typically occur during food processing.
Chemicals d-Psicose was purchased from the Rare Sugar Production Technical Research Laboratories, LLC (Kagawa, Japan). Unless otherwise specified, all other sugars and chemicals were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). HPLC-grade water was generated using a Milli-Q water system (Millipore S.A.S., Molsheim, France). Foodstuffs were purchased from local markets in the Takamatsu area, Kagawa, Japan.
Analysis The sugar content of the food products, caramel solution, and Maillard solution was determined using a post-column HPLC system, which used a gel permeation column in the ligand exchange mode (GL-C611 column [10.7 mm i.d. × 300 mm]; Hitachi, Ltd., Tokyo, Japan) coupled with a pulsed amperometric detector (Oshima et al., 2006). The brown color intensity achieved in the caramelization and Maillard reaction solutions was measured using a U-2001 spectrophotometer (Hitachi, Ltd.) at 420 nm at room temperature. The pH of each sample was obtained using a compact pH meter B-212 (Horiba, Ltd., Kyoto, Japan).
The heating procedure used in caramelization and the Maillard reaction For the caramelization of sugar (d-psicose, d-fructose or d-glucose), 3 mL each of the 40% (w/w) sugar solutions was placed in 4-mL screw-cap tubes and heated in a block heater at 60, 80, and 100°C for 24 h. For the Maillard reaction of sugar, 3 mL of an equimolar (0.05 M) mixture of each sugar and glycine was placed in 4-mL screw-cap tubes and heated in a block heater at 60, 80, and 100°C for 120 min. At specific times, each tube was removed from the heater and immediately cooled on ice. After cooling, the sugar content, brown color intensity, and final pH of the solutions were analyzed using the methods described above. The initial pH of the reaction mixture was adjusted using the following buffers (this methodology is a modified version of the procedure used by Ajandouz et al. [2001]): 0.05 M sodium acetate buffer with 1 M acetic acid at pH 4.0, 0.05 M sodium phosphate buffer at pH 6.5, 0.05 M Tris buffer with 1 M hydrochloric acid at pH 9.0, and 0.05 M sodium carbonate buffer with 1 M sodium hydroxide at pH 11.0.
Culinary treatment of food products Fig jam was cooked by heating over a low flame while the ingredients (500 g of peeled and sliced figs, 350 g of d-psicose, 35 mL of lemon juice, and 100 mL of water) were stirred in a pan for approximately 20 min until a total weight of 600 g was achieved. Nori-tsukudani was cooked by heating over a low flame while the ingredients (2 g of baked laver, 6 g of d-psicose, 10 g of soy sauce, and 80 g of water) were mixed in a beaker for approximately 50 min. Gyuhi was treated as follows: Rice flour (50 g), water (100 g), and d-psicose (50 g) were placed in a bowl and mixed. The bowl was covered with plastic wrap and heated in a microwave oven (500 W) for 1 min; the heated dough was then mixed. This process was repeated five times. Sponge cake was prepared as follows. Egg yolk (60 g), sucrose (30 g), and d-psicose (30 g) were mixed using a hand mixer. Egg white (120 g), sucrose (15 g), and d-psicose (15 g) were whipped using a KitchenAid mixer. The two mixtures were combined, and wheat flour (90 g) and melted butter (30 g) were added to the combined mixture. The resulting cake batter (350 g) was placed in a coated tube pan and heated at 180°C in a bakery oven (Scorb-4.5MP, Nichiwa Electric Co. Ltd., Hyogo, Japan) for 28 min. The meringue was produced according to the following recipe: egg white (30 g) was whipped using an electric mixer and 40 g of d-psicose was gradually added. Ten g of the meringue mixture was then placed in an aluminum cup. The uncovered cups were heated at 100°C in a dry sterilizing oven (SP-650, Toyo Seisakusho Kaisha, Ltd., Chiba, Japan) for 2 h. The d-psicose concentration and pH of each sample were determined as described above.
Stability of d-psicose during caramelization The effect of temperature on the stability of a 40% (w/w) solution of d-psicose during caramelization was examined at 60, 80, and 100°C for 24 h with a pH adjusted to 6.5 (Fig. 1). Increasing the reaction temperature in the heated solutions led to decreased levels of d-psicose. This decrease occurred gradually at 80°C and 100°C, and the residual ratio of d-psicose after 24 h at 100°C was 84.1%. d-Psicose was largely unaltered at 60°C (Fig. 1A). Moreover, raising the reaction temperature led to an increase in the brown color intensity accompanied by a decrease in the final pH. Although the brown color intensity and final pH were changed markedly at 80 and 100°C, no changes occurred at 60°C (Fig. 1B, C).

The effect of temperature on d-psicose stability during caramelization. A solution of d-psicose (40% [w/w], adjusted to an initial pH of 6.5) was heated at 60, 80, and 100°C for 24 h. A, d-Psicose levels; B, Browning; C, Final pH.
The effect of pH on the stability of a 40% (w/w) solution of d-psicose during caramelization was examined at 100°C for 24 h. The initial pH of the reaction mixtures was adjusted to 4.0, 6.5, 9.0, and 11.0 (Fig. 2). d-Psicose levels were decreased in the heated solutions as initial pH increased. At initial pH 11.0, the residual ratio of d-psicose decreased rapidly during the early stage of the process and then leveled off at approximately 60% after 8 h of incubation (Fig. 2A). Raising the initial pH in the reaction mixture led to an increase in the brown color intensity. In particular, the brown color intensity after incubation for 24 h at initial pH 11.0 was much higher than that at the other tested pHs (Fig. 2B). In the analysis at initial pH 6.5, 9.0, and 11.0, the final pH in the reaction mixture dropped in the early stages and stabilized at around pH 4 after incubation for 8 h. Conversely, at initial pH 4.0, the final pH value did not change during the reaction (Fig. 2C). De Bruijn et al (1987) reported that the drop in pH was due to an increase in acid concentrations during caramelization. Therefore, it appears that the formation of acids decreased the rate of d-psicose degradation after 8 h in the heated solution at initial pH 11.0.

The effect of pH on the stability of d-psicose during caramelization. Solutions of d-psicose (40% [w/w], adjusted to initial pH values of 4.0, 6.5, 9.0, and 11.0) were heated at 100°C for 24 h. A, d-Psicose levels; B, Browning; C, Final pH.
These results showed that the stability of d-psicose during caramelization was greatly affected by temperature and pH. Higher reaction temperatures and higher initial pHs resulted in higher levels of d-psicose degradation, browning, and a decrease in final pH with longer heating times. These behaviors were observed with other hexoses. Fig. 3 shows the stabilities of d-fructose and d-glucose under the same conditions as d-psicose. d-Fructose and d-glucose were used as examples of ketohexose and aldohexose, respectively. d-Fructose and d-glucose exhibited behaviors similar to d-psicose; these sugars were decreased in the heated solutions as the initial pH increased. Moreover, the residual ratios of these sugars were almost equivalent to that of d-psicose at all conditions tested. In addition, several researchers have reported these behaviors under various conditions. In 0.05 M fructose solutions heated at 100°C, raising the pH led to increases in fructose degradation and brown coloration and decreased pH (Ajandouz et al., 2001). In 20% fructose solutions heated at 100 – 150°C, the fructose concentration and final pH decreased with increases in the heating temperature and time (Woo et al., 2011). Luecke and Bell (2010) reported that tagatose degradation in 0.05 M tagatose solutions heated at 60 – 80°C occurred at higher reaction temperatures and higher pH and was accompanied by stronger brown color intensity. Moreover, these authors reported that the neutral pH of the tagatose solution became acid over a period of 6 to 7 days at 60°C. Thus, the reaction of d-psicose during caramelization might be not specific to d-psicose and rather might be typical of hexoses in general.

The effect of pH on the stability of d-fructose and d-glucose during caramelization. Solutions of d-fructose or d-glucose (40% [w/w], adjusted to initial pH values of 4.0, 6.5, 9.0, and 11.0) were heated at 100°C for 24 h. A, d-Fructose levels; B, d-Glucose levels.
Stability of d-psicose during the Maillard reaction The effect of temperature on the stability of d-psicose during the Maillard reaction was examined at 60, 80, and 100°C for 24 h with an equimolar (0.05 M) mixture of d-psicose and glycine with pH adjusted to 6.5 (Fig. 4). The effect of temperature on d-psicose stability is shown in Fig. 4. Increasing the reaction temperature of the heated solutions led to a decrease in the d-psicose concentration. The d-psicose decrease in each heated solution occurred gradually over time, with the d-psicose residual ratio at 100°C for 120 min being 83% (Fig. 4A). The brown color intensity increased as the reaction temperature was raised, but was largely unchanged in each solution (Fig. 4B). The final pH decreased slightly during the experimental period as the reaction temperature increased (Fig. 4C).

The effect of temperature on the stability of d-psicose during the Maillard reaction. A reaction mixture of d-psicose and glycine (0.05 M each, adjusted to an initial pH of 6.5) was heated at 60, 80, and 100°C for 120 min. A, d-Psicose levels; B, Browning; C, Final pH.
The effect of pH on the stability of d-psicose during the Maillard reaction was examined at 100°C for 24 h with an equimolar (0.05 M) mixture of d-psicose and glycine; the initial pH of the reaction mixture was adjusted to 4.0, 6.5, 9.0, and 11.0 (Fig. 5). The d-psicose residual ratio in the heated solution gradually decreased with heating time in the initial pH range of 4.0 to 9.0. The d-psicose decrease at initial pH 11.0 occurred rapidly in the early stage of the process, and after incubation at 100°C for 120 minutes, the d-psicose residual ratio became 15.2% (Fig. 5A). The brown color intensity increased linearly in the heated solution at pH 11.0, but the intensity did not change substantially in the pH range of 4.0 to 9.0 (Fig. 5B). Regarding the change in pH during the experimental period, the pH of a heated solution with an initial pH of 11.0 gradually decreased, whereas the pH did not change substantially for initial pHs ranging from 4.0 to 9.0 (Fig. 5C).

The effect of pH on the stability of d-psicose during the Maillard reaction. Reaction mixtures of d-psicose and glycine (0.05 M each, adjusted to initial pH values of 4.0, 6.5, 9.0, and 11.0) were heated at 100°C for 120 min. A, d-Psicose levels; B, Browning; C, Final pH.
These results show that the stability of d-psicose during the Maillard reaction is greatly affected by temperature and pH: higher reaction temperatures and higher initial pH facilitate d-psicose degradation, browning, and a slight decrease in final pH over time. These behaviors were observed with other hexoses. Fig. 6 shows the stabilities of d-fructose and d-glucose under the same conditions as d-psicose. d-Fructose and d-glucose were used as examples of ketohexose and aldohexose, respectively. d-Fructose and d-glucose exhibited similar behaviors to d-psicose; the concentrations of these sugars decreased in the heated solution as the initial pH increased. The residual ratio of d-fructose was almost equivalent to that of d-psicose at all conditions tested. In contrast, the residual ratio of d-glucose at initial pH 11.0 was slightly lower than that of d-psicose. In addition, these behaviors have been reported by several researchers under various conditions. In 0.05 M fructose-lysine solutions heated at 100°C, raising the initial pH led to increases in fructose degradation and brown color intensity, and decreased the final pH (Ajandouz et al., 2001). In a 0.2 M tagatose and 0.2 M glycine solution, the brown color intensity increased with increasing time, temperature, and pH (Ryu et al., 2003). Thus, the reaction of d-psicose in the Maillard reaction might not be specific to d-psicose and may be a typical reaction of ketohexoses.
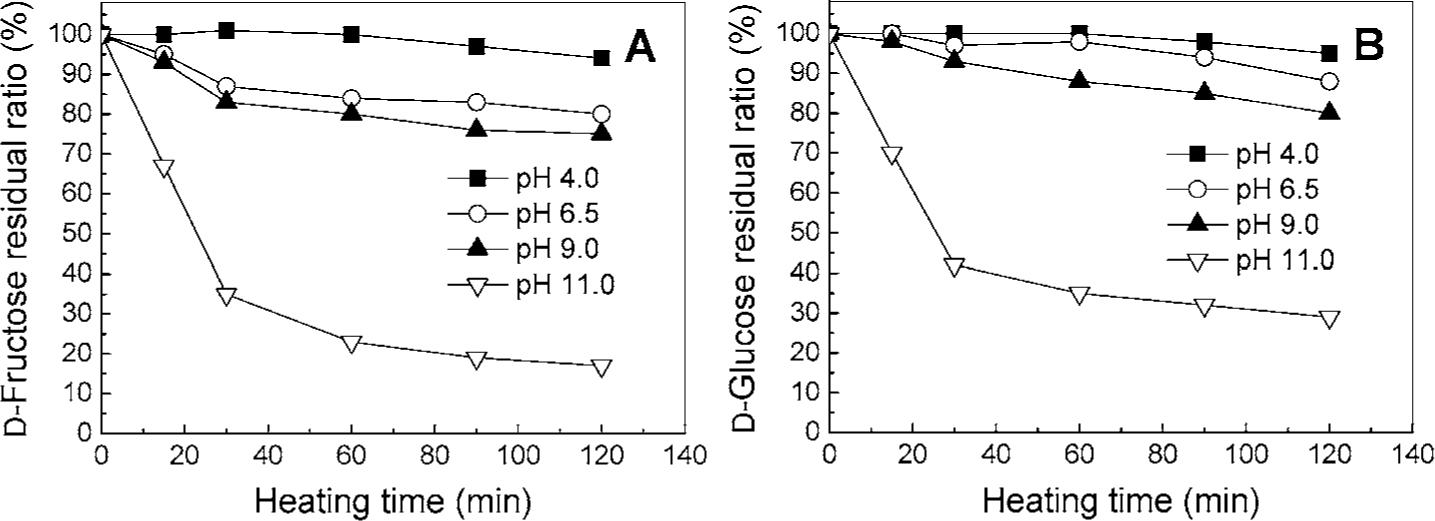
The effect of pH on the stability of d-fructose and d-glucose during the Maillard reaction. Reaction mixtures of the sugar (d-fructose or d-glucose) and glycine (0.05 M each, adjusted to initial pH values of 4.0, 6.5, 9.0, and 11.0) were heated at 100°C for 120 min. A, d-Fructose levels; B, d-Glucose levels.
Stability of d-psicose during food processing During caramelization and the Maillard reaction, raising the reaction temperature and initial pH in the heated solutions led to decreases in d-psicose, increases in the brown color intensity, and decreases in the final pH. However, other foods fortified with d-psicose may behave differently from these model solutions. Therefore, to evaluate the stability of d-psicose in general foods, we measured the d-psicose concentration and pH during the processing of foods of various pH values and under various cooking conditions.
The pH and d-psicose concentration before and after food processing are shown in Table 1. In fig jam and nori-tsukudani, which were selected as examples of acidic foods, the pH of the sample was largely unchanged, and the d-psicose concentration was decreased by only 3.3% and 3.6%, respectively. d-Psicose was thought to be quite stable during the cooking process in these samples because the heating temperature was low and the samples were acidic. Gyuhi and sponge cake were selected as models of neutral foods. The pH of gyuhi was largely unchanged, whereas the pH of sponge cake decreased from 7.8 to 6.0 during the cooking process. The d-psicose concentrations in gyuhi and sponge cake were decreased by 4.4% and 10.8%, respectively. Moreover, the gyuhi did not develop brown coloration, while the surface of the sponge cake did (data not shown). During the cooking process, the gyuhi was heated in a microwave oven (500 W) for 5 min (in total), and the sponge cake was heated at 180°C for 28 min. Therefore, the difference in the level of d-psicose decrease may be due to the heating temperatures and times used during cooking. Moreover, the major component of gyuhi is starch, which contains few amino compounds. Therefore, the composition of gyuhi may have suppressed the decrease in the level of d-psicose during its cooking. In meringue, which was selected as an alkaline food example, the pH was decreased from 8.2 to 6.0, and the d-psicose concentration was decreased by 7.7%. Moreover, the meringue developed brown coloration due to the thermal processing (data not shown).
| Sample | pH | Psicose (g/100 g)1) | Psicose loss (%) | ||
|---|---|---|---|---|---|
| Before cooking | After cooking | Before cooking | After cooking | ||
| Fig jam | 3.7 | 3.7 | 82.7 ± 3.4 | 80.0 ± 6.9 | 3.3 |
| Nori-tsukudani | 5.1 | 4.7 | 74.5 ± 1.2 | 71.8 ± 1.6 | 3.6 |
| Gyuhi | 6.5 | 6.2 | 50.0 ± 1.4 | 47.8 ± 1.2 | 4.4 |
| Sponge cake | 7.8 | 6.0 | 16.6 ± 1.5 | 14.8 ± 1.6 | 10.8 |
| Meringue | 8.2 | 6.0 | 91.9 ± 1.1 | 84.8 ± 0.6 | 7.7 |
1) Expressed as d-psicose content/100 g of dry weight. The values represent the means of three replicates ± standard deviations.
These results demonstrate the stability of d-psicose during caramelization and the Maillard reaction. That is, d-psicose was stable at lower pH, lower temperatures, and shorter heating times. Furthermore, the d-psicose was decreased in strongly heated foods that develop a brown coloration.
During caramelization and the Maillard reaction, d-psicose was degraded at increased reaction temperatures and pH. Decreases in d-psicose concentrations were accompanied by browning and decreases in pH. During food processing, similar tendencies were observed. In acidic foods, such as fig jam and nori-tsukudani, d-psicose was stable. In neutral foods, the d-psicose concentration decreased after heating at high temperature for a long period (e.g., for the sponge cake), while the d-psicose concentration did not change after heating for short periods (e.g., for the gyuhi). In alkaline foods such as meringue, the d-psicose concentration decreased as the pH decreased during heating.
These results suggest that the decrease in the d-psicose concentration that results from heating must be considered when d-psicose is added to neutral and/or alkaline foods before cooking or baking. However, d-psicose can be used effectively in many food products that are acidic and/or neutral. Moreover, controlling the temperature and pH during cooking can reduce the d-psicose loss.
Acknowledgments This work was supported in part by a grant from the Regional Innovation Cluster Program (City Area Type) of the Kagawa Industry Support Foundation and the Technical Development Association Project (Glyco-Bio-Cluster) of the Kagawa Prefectural Government, Japan.