2014 Volume 20 Issue 4 Pages 749-753
2014 Volume 20 Issue 4 Pages 749-753
To reduce the amount of salt added during the production of takanazuke, two isolated bacterial strains, B17-4 (Lactobacillus (para) plantarum) and C120-3 (Pediococcus parvulus), were used as starter strains. Takanazuke with bacterial starter was incubated with 2% (w/w) NaCl, and 6% (w/w) NaCl was added to takanazuke without starter as a control. Takanazuke was incubated at the average monthly temperatures in the Aso region (10.8 – 25.0°C). pH decreased to 4.3 with strain B17-4 and 4.2 with strain C120-3 after 12 days, while it decreased gradually to 4.2 without a starter strain after 40 days. Strain B17-4 produced less lactate and assimilated less reducing sugar than strain C120-3, which seemed to depend on the production of extracellular polysaccharide by strain B17-4. In takanazuke with starter strains and 2% (w/w) NaCl, both B17-4 and C120-3 were dominant in culture during the 181-day study.
Takanazuke, a preserved food made from takana (Brassica juncea), is produced in the Aso region of Kumamoto Prefecture as both a regional specialty and a common preserved food. At the start of takanazuke production and during the fermentation period, NaCl is added to prevent microbial contamination. When preparing long-term takanazuke to be preserved and eaten after the autumn season, 8% (w/w) NaCl is added at the start of production. This typically gives takanazuke a high NaCl concentration. Since the intake of dietary NaCl in Japan is higher than is optimal, reducing salt intake should be considered (Webster et al., 2011). Takanazuke is one such food in which dietary salt content should be reduced. Lactic acid bacteria (LAB) play an important role in the fermentation of vegetables (Hutkins, 2006), and LAB communities on vegetable leaves are dependent on conditions at harvest (Rivera-Espinoza and Gallardo-Navarro, 2010). Salt level and storage temperature during fermentation can also affect LAB communities (Tassou et al., 2002; Chao et al., 2009). The production of pickles is based on both traditional and experimental methods; however, it is difficult to estimate and regulate LAB communities during the course of natural vegetable fermentation. The traditional method is superior for the storage and safety for pickles, but it may sometimes affect taste. Sauerkraut is produced from cabbage, which also belongs to the genus Brassica, and can be produced quickly with low salt content and good taste by using starter LAB (Johanningsmeier et al., 2007; Beganovic et al., 2011). Recently, starter LAB have been used for the production of pickles from various vegetables (Yokoi et al., 2006; Yan et al., 2008; Di Cagno et al., 2011; Wouters et al., 2013), and many researchers have isolated and evaluated starter LAB for the production of sauerkraut, olive pickles, and kimchi (Bevilacqua et al., 2010; Beganovic et al., 2011; Chang and Chang, 2011; Jung et al., 2012). Use of these starter LABs can quickly decrease the pH of pickles, prevent contamination, and maintain taste, while decreasing the amount of added salt.
We previously isolated 8 LAB strains from takanazuke in 2010. Among them, 2 strains decreased pH and produced lactate rapidly in both takana juice medium and MRS medium (Sakai et al., 2013). In this study, the isolated LAB were used as starter strains for the production of takanazuke, and changes in pH and concentrations of lactate and reducing sugars were investigated during fermentation. The stability of the inoculated LAB microbial community was also determined.
Starter strains and their culture conditions B17-4 (Lactobacillus (para)plantarum) and C120-3 (Pediococcus parvulus) were used as starter strains. They produced high concentrations of lactate from takana juice medium and MRS medium (peptone 10 g/L, meat extract 10 g/L, yeast extract 5 g/L, glucose 20 g/L, Tween 80 1 g/L, K2HPO4 2 g/L, sodium acetate 5 g/L, diammonium hydrogen citrate 2 g/L, MgSO4·7H2O 0.2 g/L, and MnSO4·nH2O 0.05 g/L) while decreasing pH (Sakai et al., 2013). Both strains were cultivated on MRS plates containing 15 g/L of agar at 30°C for 48 h. A loopful of LAB was inoculated in 30 mL of MRS medium and precultured at 24°C for 24 h. Twenty milliliters of precultured medium was inoculated in 200 mL of new MRS medium and incubated statically at 30°C for 24 h.
Production of takanazuke with starter LAB Takana was harvested in April 2011 from Ichihara Farm in Aso City, Kumamoto Prefecture and either 2% (w/w) NaCl or 6% (w/w) NaCl was added. Specimens were later brought back to Kumamoto City, which was recorded as day 0 of our study. One kilogram each of 2% (w/w) NaCl takana was inoculated with either 1.0 × 1010 cfu of B17-4 or 4.3 × 1010 cfu of C120-3 on day 0. No LAB was inoculated in the 6% (w/w) NaCl takana. The 2% (w/w) NaCl takana with starter LAB was divided into 60 g portions and sealed by vacuum sealer. Control consisting of 6% (w/w) NaCl takana with no starter LAB was divided into 110 g portions and was incubated after sealing. All takana was incubated at the average monthly temperatures of the previous year (2010) for the Aso region as follows: days 1 – 28 (April) at 10.8°C; days 29 – 59 (May) at 15.9°C; days 60 – 89 (June) at 20.0°C; days 90 – 120 (July) at 23.6°C; days 121 – 151 (August) at 25.0°C; and days 152 – 181 (September) at 21.8°C. No water was removed from sealed 2% (w/w) NaCl takana with starter LAB, while 3.9, 4.3, 3.8, 3.9, and 3.4% (w/w) of water was removed from the continuously incubated control 6% (w/w) NaCl takana without starter LAB on days 2, 4, 6, 17, and 31, after which the control was resealed.
Analysis of exopolysaccharides and component sugars A loopful of B17-4 was inoculated in 10 mL of MRS medium and incubated at 30°C for 24 h. Five milliliters of precultured medium was inoculated in 50 mL of MRS medium and incubated at 30°C for 24 h. Three milliliters of cultured medium was centrifuged at 8000 × g at 4°C for 15 min. After the supernatant was removed, 3 mL of sterilized water was added to the precipitate, B17-4. The suspended B17-4 was inoculated in 300 g of 2% (w/w) NaCl takana and incubated at 30°C for 7 days. Exopolysaccharides (EPS) were extracted following the method of Salazar et al., 2009. Briefly, prepared takanazuke was wrung with a triple layer of gauze, and the obtained solution was mixed with an equal volume of 1 N NaOH solution and kept at room temperature with mixing overnight. The solution was then centrifuged at 8000 × g at 4°C for 15 min. A two-fold volume of cold ethanol was added to the supernatant and kept at 4°C for 48 h. The sample was then centrifuged at 10,000 × g at 4°C for 30 min and the precipitate was rinsed with ethanol and centrifuged again. The resulting precipitate was air-dried and dissolved in 3 mL of pure water. From this sample, 2.7 mL was mixed with 0.3 mL of 25% HCl solution and boiled for 3 h. The sample was cooled to room temperature, and its pH was adjusted to 5 – 6 by the addition of 10% NaOH solution, bringing its volume to 4 mL. After filtration using a 0.45-µm acetate filter, the sample was analyzed by high-performance liquid chromatography (HPLC) as described in Analysis.
Determination of the microbial community of takanazuke with starter strains Prepared takanazuke was wrung with a triple layer of gauze. The obtained solution was centrifuged at 8000 × g at 4°C for 15 min and the precipitate was suspended in sterilized water. The suspension was centrifuged again at the same settings and the precipitate was suspended in 200 µL of sterilized water. The bead beating method was then used to extract DNA. The extracted DNA was used as a template for PCR, targeting partial 16S rRNA gene sequences. The primer set was 27F (5'-AGAGTTTGATCCTGGCTCAG-3') and 518R (5'-GTATTACCGCGGCTGCTGG-3'). AmpliTaq Gold (Applied Biosystems, Carlsbad, CA) was used for PCR. After preheating at 95°C for 5 min, 25 cycles of heating at 95°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 2 min were performed. PCR products were purified by an Ultra Clean PCR Clean-up Kit (MO-BIO, Carlsbad, CA) according to the manufacturer's protocol. Purified PCR products were ligated with pT7Blue vector (Novagen, Darmstadt, Germany) by using a Ligation Mix Kit (Takara, Kyoto, Japan) according to the manufacturer's protocol. Then, 100 µL of competent Escherichia coli DH5α cells (Takara) was transformed with 10 µL of ligation mixture by conventional methods. After colonies formed on an LB-ampicillin plate, white colonies were selected and plasmids were extracted using the Wizard SV Minipreps DNA Purification System (Promega, Madison, WI). Extracted plasmids were digested by EcoRI (Takara) and PstI (Takara) to determine the size of the inserted DNA. Sequence analysis of the inserted DNA was completed by Takara Co., Ltd. Data from the analysis were compared to the NCBI database by a BLAST search to identify obtained clones.
Analysis Prepared 2% (w/w) NaCl takanazuke with starter LAB was wrung with a triple layer of gauze after incubation for 0, 3, 7, 12, 30, 60, 90, and 181 days. Takana on day 0 was wrung before LAB addition. Control 6% (w/w) NaCl takanazuke without starter LAB was wrung after 0, 4, 7, 19, 30, 40, 90, and 180 days. The obtained solution was also used for measurement of pH, lactate concentration, and reducing sugar concentration after centrifugation at 8000 × g at 4°C for 15 min and following filtration by 0.45 µm cellulose acetate filter (ADVANTEC). pH was analyzed by a pH meter (HM-25G; DKK-TOA, Tokyo, Japan). D/L lactate concentration was analyzed by F-kit lactate (Roche Diagnostics Corporation, Basel, Switzerland). Concentration of reducing sugar was determined by the Somogyi-Nelson method. Concentration of sugars such as arabinose, mannose, galactose, xylose, and glucose was analyzed by HPLC (LC-10A System, Shimadzu, Kyoto, Japan) using a Shim-pack ISA-07/S2504 column (Shimadzu), and linear gradient elution with potassium borate buffer as mobile phases A (0.1 M) and B (0.4 M). Total flow rate of the mobile phase solution was 0.6 mL/min and the column was kept at 65°C. Sugar was detected by fluorescence with excitation at 320 nm and emission at 430 nm after reaction with 1% (w/v) arginine and 3% (w/v) borate at 150°C. Standard solution of sugars was purchased from Sigma-Aldrich, Japan. Viable LAB were counted as colonies on the MRS plate after a series of dilutions of cultured medium were plated and incubated at 30°C for 3 days. All analyzed data were obtained by single experiment.
Changes in pH during fermentation Changes in pH of the solution obtained from takanazuke are shown in Fig. 1. Takanazuke with B17-4 or C120-3 starter strains showed a rapid decrease in pH over the first 7 days. The pH values of B17-4 and C120-3 takanazuke on day 3 were 6.0 and 6.6, respectively, on day 7 both were decreased to 4.7, and on day 12 were decreased again to 4.3 and 4.2, respectively. After 181 days, pH decreased to 4.1 and 3.8, respectively. The pH of the control takanazuke decreased gradually to 6.5, 6.1, and 5.8 after 4, 7, and 19 days, respectively. The pH further decreased to 4.2 after 40 days and finally to 3.8 after 180 days. The pH change of takanazuke fermented under the conditions with 2% (w/w) NaCl and without LAB addition at Ichihara farm in Aso city was 6.7 on day 4, 6.0 on day 15, and 4.0 on day 46. Its pH changes were similar to those seen in the control takanazuke.

Changes in pH during fermentation. Symbols: □, B17-4; △, C120-3; ◆, control; ×, Ichihara Farm.
In Spanish-style green olive fermentation, pH has been shown to decrease when including a starter strain of L. plantarum LPCO10 during the first 10 days; however, it has also been shown to decrease to the same value after 60 days even without starter LAB (Leal-Sanchez et al., 2003). In the present takanazuke fermentation, both B17-4 and C120-3 decreased the solution pH rapidly in the same manner as green olive fermentation, to almost identical levels.
Changes in lactate concentration Changes in lactate concentration during takanazuke fermentation are shown in Fig. 2. Lactate concentrations rapidly increased during the first 12 days for both starter strains. However, on day 12 of fermentation, the lactate concentration in the B17-4 sample was 18.6 g/L, which was lower than the 21.9 g/L seen in the C120-3 sample. After 12 days, lactate concentrations in the B17-4 sample were still lower than those in the C120-3 sample. In the control sample, lactate concentration increased during fermentation and after 180 days almost reached the level of the C120-3 sample. The final concentrations on day 180 and 181 with B17-4, C120-3, and for the control were 24.5 g/L, 30.5 g/L, and 28.8 g/L, respectively. Ratios of l-lactate to whole lactate with B17-4, C120-3, and for the control were 28.0%, 51.1%, and 42.7%, respectively. As organic acids can inhibit the growth of bacteria and fungi (Holzapfel, 2002), both B17-4 and C120-3, which produced lactate rapidly, could prevent contamination during the first stage of fermentation.

Changes in lactic acid concentration during fermentation. Symbols: □, B17-4; △, C120-3; ◆, control.
Changes in reducing sugar concentration Concentrations of reducing sugar were determined since lactate concentration between the B17-4 and C120-3 samples differed. The initial concentration of reducing sugar was 33.5 g/L on day 0. On day 7, when pH decreased to 4.7, reducing sugar concentration decreased to 27.0 g/L and 24.0 g/L in the B17-4 and C120-3 samples, respectively. After day 7, concentrations of reducing sugar in the B17-4 sample were higher than those in the C120-3 sample, as shown in Fig. 3. Supernatant sugar composition on day 181 is shown in Table 1. Total concentration of sugars in the B17-4 and C120-3 samples was 3.89 g/L and 3.57 g/L, respectively, and concentrations of reducing sugars of the same sample were 9.49 g/L and 5.29 g/L, respectively. Therefore, the concentration of sugars other than arabinose, mannose, galactose, xylose, and glucose in the B17-4 sample was higher than that in the C120-3 sample.
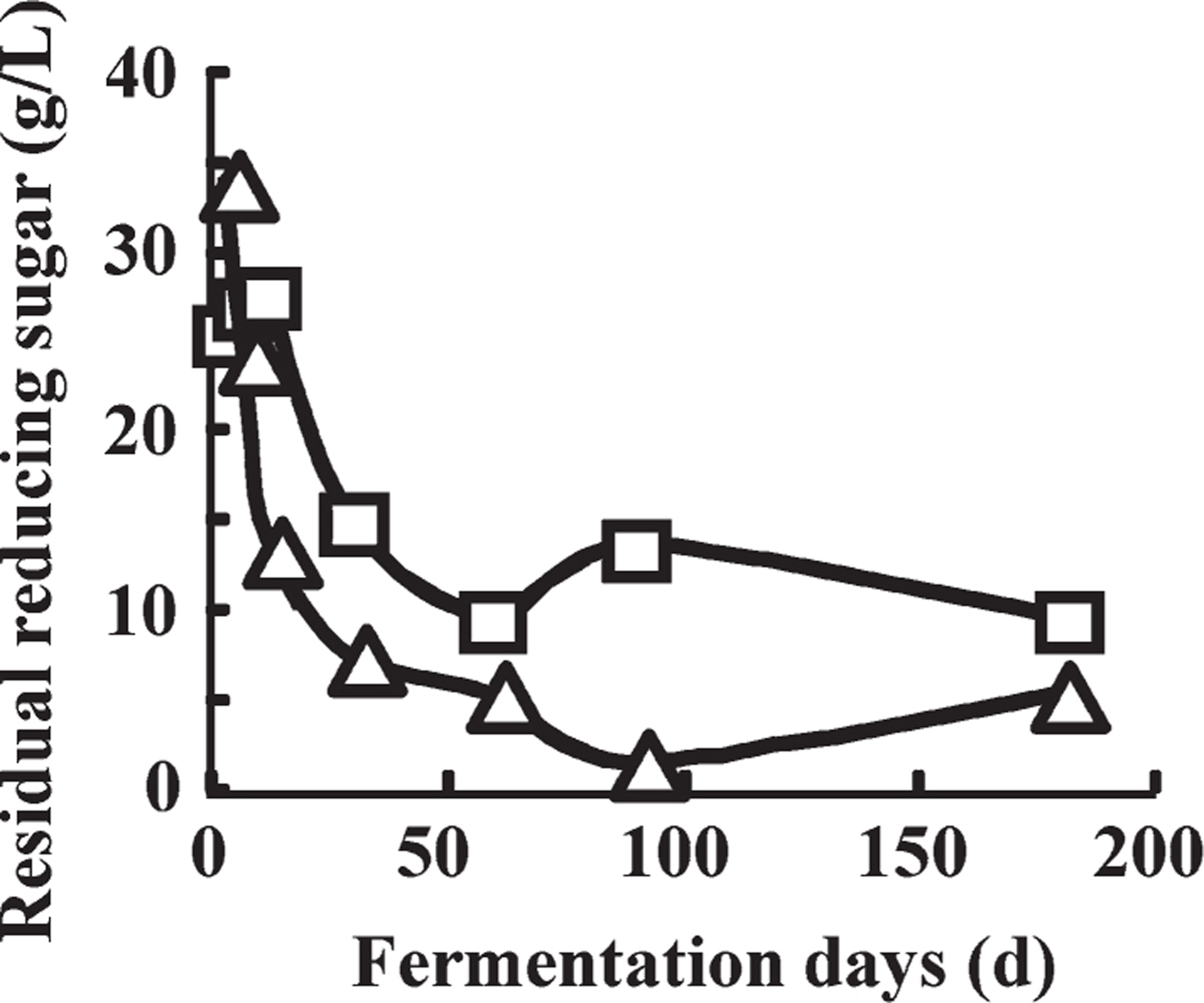
Changes in residual reducing sugar concentration during fermentation. Symbols: □, B17-4; △, C120-3.
| B17-4 | C120-3 | |
|---|---|---|
| Arabinose | 3.00 | 0.51 |
| Mannose | 0.00 | 0.08 |
| Galactose | 0.52 | 0.67 |
| Xylose | 0.03 | 0.04 |
| Glucose | 0.34 | 2.27 |
| Others | 5.60 | 1.72 |
| Total | 9.49 | 5.29 |
Production of EPS by B17-4 The solution obtained during fermentation of takanazuke with B17-4 seemed to be highly viscous. This secreted compound could be observed on the MRS plate with B17-4, but not on the MRS plate with C120-3. Some L. plantarum have been reported to be able to produce EPS from sugars contained in the medium (Tallon et al., 2003; Liu et al., 2011). Here, B17-4 also produced EPS during fermentation. It has also been reported that L. rhamnosus R produces EPS, which it can degrade to a monosaccharide by using its own glycohydrolases (Pham et al., 2000; Badel et al., 2011). However, many EPS-producing LABs cannot degrade EPS to monosaccharides with their own enzymes (Wolfaadt et al., 1999). It seemed that here, B17-4 produced EPS from the early stages of fermentation and could not use its own glycohydrolases to degrade these EPSs to monomers.
We confirmed that B17-4 could produce EPS in a model takanazuke fermentation test. Produced EPS was hydrolyzed by acid and its sugar composition was determined by HPLC. As shown in Table 2, the major components were arabinose, galactose, and glucose. It showed a similar composition to the sugars shown in Table 1.
| Sugar | Content |
|---|---|
| Arabinose | 26 |
| Mannose | 5 |
| Galactose | 30 |
| Xylose | 2 |
| Glucose | 37 |
| Total | 100 |
Stability of microbial community in takanazuke with starter LAB DNA was extracted from takanazuke with B17-4 and C120-3 on days 12, 90, and 181. More than 20 clones were determined by homology analysis and obtained data are shown in Table 3. In takanazuke with B17-4, all clones were most similar to L. paraplantarum AB626065 and L. plantarum HQ610929, which are the closest relatives to B17-4. In takanazuke with C120-3, clones obtained until day 90 were most similar to P. parvulus AB681216, which is the closest relative to C120-3. However, on day 181, two clones other than P. parvulus AB681216 were also detected. One was most similar to Leuconostoc mesenteroides AB596937, a heterotype of LAB, and the other was most similar to Schlegelella sp. KB1a AY538706, which was originally isolated as a thermotolerant poly (3-hydroxybutyrate) (PHB)-degrading bacteria (Romen et al., 2004). Starter strains were dominant during fermentation in both takanazuke with B17-4 and with C120-3. When takanazuke is preserved for a long period, such as over the summer, the salt content will generally rise.
| B17-4 | C120-3 | |||||
|---|---|---|---|---|---|---|
| Day | Closest relatives | Number of clones | Sequence similarities | Closest relatives | Number of clones | Sequence similarities |
| Day 12 | L. paraplantarum AB626065 L. plantarum HQ610929 |
20/20 | 100 – 99.8% | P. parvulus AB681216 | 24/24 | 100 – 99.8% |
| Day 90 | L. paraplantarum AB626065 L. plantarum HQ610929 |
21/21 | 100 – 99.8% | P. parvulus AB681216 | 23/23 | 100 – 99.8% |
| Day 181 | L. paraplantarum AB626065 | 22/22 | 100 – 99.8% | P. parvulus AB681216 | 21/23 | 100 – 99.8% |
| L. plantarum HQ610929 | Leu. mesenteroides AB596937 | 1/23 | 100% | |||
| Schlegelella sp. KB1a AY538706 | 1/23 | 99.8% | ||||
We found that takanazuke could be produced with a low level of added salt by using starter LAB. Starter LAB inoculation can also prevent contamination in a 2% (w/w) salt environment for up to 181 days. Produced lactic acid by starter LAB could prevent contamination during the first stage of fermentation. The change in the microbial community of control takanazuke is under investigation and will be reported in the near future.
Acknowledgements We thank Mr. Tsuyoshi Ichihara of Ichihara Farm in Aso City for his kind contribution of takana and for his much appreciated advice on the production of takanazuke.