2016 Volume 22 Issue 2 Pages 235-243
2016 Volume 22 Issue 2 Pages 235-243
Proteins contained in the green leaf mucus of Japanese bunching onion (Allium fistulosum L.) were isolated by ammonium sulfate precipitation and separated by hydrophobic interaction chromatography. The mucus proteins had an enhanced effect on tumor necrosis factor (TNF)-α production in the RAW 264 murine macrophage cell line. Protein composition was analyzed by SDS-polyacrylamide gel electrophoresis and N-terminal amino acid sequencing. The effect on RAW 264 cells was mainly attributed to mannose-binding lectin and thaumatin-like proteins, which occupied 34% of the mucus proteins. Other major proteins contained in the mucus showed homologous amino acid sequences to those of lachrymatory factor synthase, β-1,3-glucanase, 2S albumin precursor, and pathogenesis-related protein 4. Protein composition ratios in the mucus of 13 cultivars of Japanese bunching onion were also examined. Taken together, the green leaf mucus of Japanese bunching onion may be a possible source of various bioactive proteins.
Japanese bunching onion (Allium fistulosum L. (Liliaceae)), also called Welsh onion, is a perennial onion and widely cultivated throughout world, especially in East Asia. It has two edible flavored portions, the green leaves and the white sheaths, the proportions of which vary depending on the variety. This vegetable crop is important both economically and as an ingredient in Asian cuisine. Japanese bunching onion has also been used as a folk medicine for treating febrile disease, headache, abdominal pain, etc. (Wang et al., 2006; Duh et al., 2008). In fact, various phytochemicals including organosulfur compounds (Zhou et al., 2011), quercetin, kaempferol (Aoyama et al., 2007), and fructan (Lee et al., 2012) have been identified as active components in the plant. Japanese bunching onion secretes mucus in its hollow tube-like green leaves. The mucus was shown to increase the production of tumor necrosis factor (TNF)-α and monocyte chemotactic protein (MCP)-1 from the RAW 264 murine macrophage cell line and of interleukin (IL)-12 from J774.1 cells; however, extracts from the green leaves and white sheaths did not (Ueda et al., 2013). Oral administration of the mucus to mice augmented the immune functions of peritoneal cells by increasing TNF-α and IL-12 production and phagocytosis. It also increased interferon (IFN)-γ production from spleen cells and natural killer (NK) activity, suggesting that the mucus can enhance natural immunity (Ueda et al., 2013).
Some plant lectins, which are proteins of non-immune origin, have been shown to exert immunomodulatory activities, which are initiated by their interaction with glycan moieties present on immune cells (Van Damme et al., 1998). For example, onion (Allium cepa) lectin (ACA) significantly stimulated the production of TNF-α and IL-12 in RAW 264.7 cells and rat peritoneal macrophages (Prasanna and Venkatesh, 2015). Garlic (Allium sativum) lectins (ASA I and ASA II) also exhibited potent immunomodulatory activities in vitro and in vivo (Clement et al., 2010). Besides lectins, only a few proteins including alliinase (Van Damme et al., 1993), allicepin (Wang and Ng, 2004), and protease inhibitors (Deshimaru et al., 2003) have been isolated from Allium sp. and characterized.
In this study, lectins and other protein components contained in the green leaf mucus of Japanese bunching onion were analyzed to reveal the relationship between the protein components and the stimulatory effect of the mucus on the RAW 264 murine macrophage cell line. In addition, the protein compositions of the green leaf mucus prepared from 13 cultivars of Japanese bunching onion were examined and their lectin activities and stimulatory effects on TNF-α production in RAW 264 cells were measured.
Materials Thirteen cultivars of Japanese bunching onion (A. fistulosum) were cultivated at the National Institute of Vegetable and Tea Science in Mie Prefecture, Japan. The cultivars used were Amarumeipponbuto, Shimonita, Jionji, Yoshikura, Omiyaguro, Nishida, Kiyotaki, Koshizuaigara, Kujobuto, Asagikeikujo, Akahige, Shokyu, and Fuyuwarabe. The harvested plants were weighed and divided into green leaves and white sheaths. The green leaves were dipped into purified water, and the mucus was collected into water from the inner cavities of the leaves. The mucus solutions were filtered through nylon mesh sheets of 1-mm pore size and lyophilized in a tray lyophilizer (Tokyo Rikakikai, Tokyo, Japan).
HiTrap Butyl HP and HiTrap Q HP were obtained from GE Healthcare Bio-Sciences (Piscataway, NJ, USA). Polyvinylidene difluoride (PVDF) membrane was purchased from Bio-Rad Lab. (Hercules, CA, USA). RAW 264 cells (murine leukemic monocytes) were obtained from RIKEN Bio Resource Center (Tsukuba, Japan). Eagle's minimum essential medium containing 2 mM L-glutamine, penicillin G and streptomycin was from Wako Pure Chemical Industries (Osaka, Japan). Fetal bovine serum (10%) was purchased from Invitrogen Canada (ON, Canada). A mouse TNF-α enzyme-linked immunosorbent assay (ELISA) kit (Mouse TNF-α ELISA MAX™ Deluxe Set) was obtained from BioLegend (San Diego, CA, USA). Lipopolysaccharide (LPS) from Escherichia coli serotype O127:B8 was purchased from Sigma-Aldrich (St. Louis, MO, USA). WST-1 assay kit was from Dojindo Laboratories (Kumamoto, Japan). All other chemicals used in this study were of analytical grade and purchased from Wako Pure Chemical Industries or Nacalai Tesque (Kyoto, Japan).
Preparation of protein fractions Lyophilized mucus (Yoshikura cultivar) (5.0 g) was homogenized in 50 mM Tris-HCl buffer (pH 8.0) containing 0.3 M NaCl and then centrifuged at 15,000 × g for 10 min at 4°C. The protein in the supernatant was precipitated by the addition of solid (NH4)2SO4 to 80% saturation. The precipitate was collected by centrifugation, dialyzed against purified water, and lyophilized. The yields of protein fractions were 0.4–0.8% (w/w).
For further fractionation, the precipitate was dissolved in 15 mL of 50 mM Tris-HCl (pH 8.0) containing 1.0 M (NH4)2SO4. After centrifugation, the supernatant was subjected to hydrophobic interaction chromatography on a HiTrap Butyl HP column (10 mL) pre-equilibrated with 50 mM Tris-HCl (pH 8.0) containing 1.0 M (NH4)2SO4, and then eluted with a decreasing linear gradient of (NH4)2SO4 (1.0 to 0 M) in the same buffer at a flow rate of 1.0 mL/min. Each fraction was collected, dialyzed against purified water, lyophilized, and stored at 4°C until use.
The protein fractions containing mannose-binding lectin and thaumatin-like proteins (TLPs) were subjected to anion exchange chromatography on a HiTrap Q HP (5 mL) column pre-equilibrated with 50 mM Tris-HCl (pH 8.0/8.7), and eluted with a linear gradient of NaCl (0–1.0 M) in the same buffer at a flow rate of 1.0 mL/min. The major peaks were collected, dialyzed against purified water, and lyophilized. The protein fractions obtained by hydrophobic interaction chromatography were also separated by reversed-phase high-performance liquid chromatography (HPLC) on a Wakosil 5C4-200 column (5 µm, 4.6 × 250 mm) (Wako Pure Chemical Industries) using a gradient of acetonitrile in 0.1 % trifluoroacetic acid (TFA) at a flow rate of 1.0 mL/min
Characterization of protein fractions The molecular mass of proteins was measured by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 15% separating gel in the presence or absence of 2-mercaptoethanol (ME). Protein bands were stained with Coomassie Brilliant Blue (CBB) R-250. The proximate protein composition of each cultivar was measured by their stained band intensities on SDS-PAGE gels using the computer program CS analyzer version 3.0 (ATTO, Tokyo, Japan).
For N-terminal amino acid sequencing, proteins separated on SDS-PAGE gels were transferred onto PVDF membranes by semi-dry electroblotting (Lauriére, 1993). The protein bands were stained with CBB R-250, excised carefully, and washed thoroughly with ethanol and purified water. Each small piece of PVDF membrane, representing a protein band, was subjected to sequencing by a gas phase protein sequencer (PPSQ-10; Shimadzu, Kyoto, Japan).
For internal amino acid sequencing, purified protein was reduced with dithiothreitol (DTT) and S-carboxamidomethylated (CAM) with monoiodoacetamide. CAM-protein was digested with Achromobacter protease (Wako Pure Chemical Industries) for 18 h at 37°C. The digest was separated by reversed-phase HPLC on a TSKgel ODS 120T column (5 µm, 4.6 × 250 mm; Tosoh, Tokyo) using a linear gradient of acetonitrile in 0.1% TFA. The isolated peptide fragments were analyzed using a gas-phase protein sequencer.
Hemagglutination and inhibition assays Lectin activity was estimated by hemagglutination activity (HA) against rabbit erythrocytes. Samples (50 µL) (2-fold serial dilutions in 0.15 M NaCl) were mixed with 50 µL of 2% rabbit erythrocyte suspension in round-bottom microtiter plates (96-well) by agitation for 30 s and hemagglutination was measured after incubation for 1 h at room temperature. HA was defined as the maximum dilution (2n) with positive agglutination.
The inhibitory effects of saccharides on hemagglutination were assayed as follows. The saccharide solutions (25 µL) were diluted 2-fold in series on microtiter plates and incubated with 25 µL of the lectin solution having hemagglutination titer values of 23 for 30 min. The 2% rabbit erythrocyte suspension (50 µL) was added to the mixture and incubated for 1 h. The inhibitory activities were estimated by the minimum concentration of sugars needed to cause negative hemagglutination.
Metal ion dependency for hemagglutination was examined by the treatment with ethylenediamine-tetraacetic acid (EDTA). The lectin solution was treated with 0.1 M EDTA in 50 mM Tris-HCl containing 0.15 M NaCl (pH 7.5) at room temperature for 15 min, and then dialyzed against 0.15 M NaCl overnight at 4°C. The lectin solution was tested for HA in the presence of 50 mM Mn2+, Mg2+ and Ca2+, respectively, in 50 mM Tris-HCl (pH 8.0) containing 0.15 M NaCl.
Thermostability of the lectin activity was examined using the hemagglutination assay as described above after incubation at various temperatures (40, 45, 50, 55, 60, and 80°C) for 30 and 120 min.
To examine pH stability, the lectin solution was incubated at various pH overnight at 4°C. The buffers used were 50 mM HCl-KCl containing 0.15 M NaCl and 5 mM CaCl2 (pH 1.0), 50 mM CH3COONa containing 0.15 M NaCl and 5 mM CaCl2 (pH 3.0 and 5.0), 50 mM Tris-HCl containing 0.15 M NaCl and 5 mM CaCl2 (pH 7.0 and 9.0), and 50 mM sodium carbonate containing 0.15 M NaCl and 5 mM CaCl2 (pH 11.0 to 14.0). After adjusting the pH to 7.5 with 1.0 M HCl or 1.0 M NaOH, hemagglutination was measured as described above.
Induction of TNF-α production in RAW 264 cells RAW 264 cells were grown in Eagle's minimum essential medium containing 2 mM L-glutamine and supplemented with 10% fetal bovine serum, 100 U/mL of penicillin G, and 100 mg/mL of streptomycin (Wako Pure Chemical Industries) at 37°C in a 5% CO2 humidified incubator. The cells (1×105 cells/200 µL/well) were seeded in a flat-bottomed 96-well culture plate and precultured for 5 h. Test samples were added to each well, and the cells were cultured for 18 h. LPS (100 ng/mL) was used as a reference. The culture supernatants were then transferred to a 96-well round-bottomed plate and assayed for TNF-α by ELISA.
The cytotoxicity was measured by a WST-1 assay kit after incubating the cells in the presence of samples for 18 h. The WST-1 reagent was incubated with the cells for 1 h, and then the absorbance at 450 nm was measured by a titer plate reader (Bio Rad, Hercules, CA, USA).
Isolation of mucus proteins and lectin activity determination Yields of lyophilized mucus obtained from the raw green leaves of Japanese bunching onion (Yoshikura cultivar) were about 1% (w/w). The weight of the lyophilized mucus was reduced by 50 – 60% after the removal of small molecules by dialysis. Proteins contained in the mucus were fractionated by stepwise (NH4)2SO4 precipitation. Fractions obtained by successive precipitation with (NH4)2SO4 of 30%, 60%, and 80% saturation, and the supernatant of 80% saturated (NH4)2SO4 were desalted by dialysis and lyophilized. They were dissolved in the same volume of 50 mM Tris-HCl (pH 8.0) containing 0.15 M NaCl, and subjected to SDS-PAGE and hemagglutination assay. Stepwise precipitation with (NH4)2SO4 of 60% and 80% saturation yielded different protein compositions at the weight ratio of 5:1, as shown by the SDS-PAGE protein profiles (Fig. 1). Most proteins were precipitated with (NH4)2SO4 of 80% saturation.

SDS-PAGE patterns of mucus proteins of Japanese bunching onion (Yosikura cultivar) fractionated by (NH4)2SO4 precipitation of 30% (A), 60% (B), 80% (C) saturation in 15% gels. D: Supernatant of 80% saturated (NH4)2SO4 precipitation. −ME: Nonreduced condition, +ME: reduced condition. I: β-1,3-glucanase, II: thaumatin-like protein B, III: thaumatin-like protein A-a, IV: thaumatin-like protein A-b, V: mannose-binding lectin, VI: 2S albumin precursor, VII: lachrymatory factor synthase a, VIII: lachrymatory factor synthase b, IX: pathogenesis-related protein 4.
Hemagglutination activity (HA) of each fraction was 32, 128, 8, and 4, respectively, showing that most lectins were precipitated with 60% saturated (NH4)2SO4. The minimum concentration of the protein fraction required for HA toward rabbit erythrocytes was 80 µg/mL. HA was increased 16-fold by treating rabbit erythrocytes with trypsin (0.01% w/v) at 37°C for 1 h, but not with protease. The hemagglutination was inhibited by D-mannose at 12.5 mM. No inhibition was observed with D-glucose, D-galactose, D-fructose, N-acetyl-D-glucosamine, or N-acetyl-D-galactosamine even at a concentration of 1.0 M, indicating that the mucus contained a mannose-binding lectin. The lectin activity was lost by EDTA treatment. The addition of 50 mM Ca2+ fully recovered the activity, while Mn2+ recovered it very marginally. On the other hand, 50 mM Mg2+ did not recover the activity at all, indicating the requirement of Ca2+ for HA of the lectin.
The lectin maintained its full HA after incubation at 40°C for 120 min (Fig. 2). The activity decreased above 50°C for 120 min and was lost after heating at 80°C for 120 min. The lectin maintained full activity after incubation at pH5-12 overnight at 4°C, though the activity decreased by 50% at pH3 and pH13 (data not shown).

Effect of temperature on the hemagglutination activity of the mucus protein fraction of Japanese bunching onion (Yoshikura cultivar). The mucus protein solutions were incubated at the indicated temperatures for 5–120 min. After cooling to room temperature, the hemagglutination activity was measured.
Induction of TNF-α production in RAW 264 cells by mucus proteins The production of TNF-α in RAW 264 cells was increased by the addition of mucus. The fraction precipitated with 60% saturated (NH4)2SO4 showed much stronger activity than the 80% saturated (NH4)2SO4 fraction (Fig. 3A). The stimulatory activity decreased considerably by heat-treatment (80°C, 120 min) (Fig. 3B) at 5 µg/mL, indicating that protein components play an important role in the activity of Japanese bunching onion mucus. However, the activity was still significant at 50 µg/mL even after heat treatment. Although the lyophilized mucus showed some cytotoxicity against RAW 264 cells at 1000 µg/mL with 18-h incubation, no cytotoxicity was observed with the fraction precipitated with (NH4)2SO4 at the same concentration (data not shown).

TNF-α production in RAW 264 cells stimulated with mucus protein fractions (Yoshikura cultivar) obtained by (NH4)2SO4 precipitation of 0–60% and 60–80% saturation (A). (B) The effect of heat treatment (80°C, 120 min) of the protein fraction on TNF-α production in RAW 264 cells. Data are presented as the mean ± SD of three experiments.
Analysis of protein components The protein fraction obtained from the Yoshikura cultivar by 60% saturated (NH4)2SO4 precipitation was separated by hydrophobic interaction chromatography into fractions 1–10 (Fig. 4). The protein profiles on SDS-PAGE of each fraction are shown in Fig. 5. Each fraction, which was dialyzed against purified water and lyophilized, was subjected to the TNF-α production assay using RAW 264 cells and the hemagglutination assay using rabbit erythrocytes. Only fraction 9 revealed high HA among the fractions (Fig. 6). Meanwhile, fractions 4, 5, and 6, as well as fraction 9, showed high activities in TNF-α production.

Separation of the mucus proteins (Yoshikura cultivar) by hydrophobic interaction chromatography on a HiTrap Butyl HP column (10 mL) pre-equilibrated with 50 mM Tris-HCl (pH 8.0) containing 1.0 M (NH4)2SO4 and eluted with a decreasing linear gradient of (NH4)2SO4 (1.0 to 0 M) in the same buffer at 1.0 mL/min. The mucus protein fraction obtained by 60% saturated (NH4)2SO4 precipitation was used.

SDS-PAGE patterns of fractions 1–10 separated by hydrophobic interaction chromatography of the mucus protein (Yoshikura cultivar) on a 15% gel. Fractions 1–10 were prepared under the conditions described in Fig. 4. I: β-1,3-glucanase, II: thaumatin-like protein B, III: thaumatin-like protein A-a, IV: thaumatin-like protein A-b, V: mannose-binding lectin, VI: 2S albumin precursor.

Activities of fractions 1–10 separated by hydrophobic interaction chromatography of the mucus protein (Yoshikura cultivar). Fractions 1–10 were prepared under the conditions described in Fig. 4. The stimulatory activity of 50 µg/mL of each fraction on TNF-α production in RAW 264 cells was measured. LPS (100 ng/mL) was used as a reference. Data are presented as the mean ± SD of three experiments. Lectin activities are presented as HA of each fraction at 1.0 mg/mL. Data are presented as the mean ± SD of three experiments.
N-Terminal amino acid sequencing of mucus proteins The mucus proteins of the Yoshikura cultivar separated by SDS-PAGE were subjected to N-terminal amino acid sequencing after electroblotting onto PVDF membranes. Firstly, major bands of the protein fractions obtained by stepwise (NH4)2SO4 precipitation of 60% and 80% saturation, respectively, were analyzed (Fig. 1).
The N-terminal amino acid sequences of bands I-IX are summarized in Table 1. These amino acid sequences were searched using the BLAST program and NCBI protein databases. The N-terminal amino acid sequence of band I was homologous to that of β-1,3-glucanase from Vitis riparia (GenBank ACCESSION: AAR06588). The protein is classified as pathogenesis-related (PR) protein, PR2, and is known to hydrolyze bacterial cell walls composed of β-1,3-glucan (Sels et al., 2008). Band II showed homology to thaumatin-like protein (TLP) from Arabidopsis thaliana (BAB11214), which is classified as PR5. Bands III and IV gave the same N-terminal sequences, which were homologous to that of TLP from Vitis vinifera (AAQ10092). In this paper, band II and band III/IV were called TLP B and TLP A-a/A-b, respectively, for the sake of convenience. Band V was revealed to be a mannose-binding lectin, based on its N-terminal sequence deduced from the cDNA sequence of the lectin from A. fistulosum (LC003019, LC003020). Band VI gave no sequence information because of the blockage of the N-terminus. The N-terminal amino acid sequences of bands VII and VIII showed significant homologies to lachrymatory factor synthase from A. fistulosum (BAC22638). Bands VII and VIII were tentatively distinguished as lachrymatory factor synthase a and b, respectively, in this study. The enzyme was reported to synthesize a lachrymatory factor, propanethial S-oxide, from 1-propenylsulphenic acid (Imai et al., 2002). Band IX gave a homologous sequence to PR4 from Lycoris radiata (ACI31201). All PR4 have a common C-terminal Barwin domain that is considered to act as chitinase (Sels et al., 2008).
| I:IGVXYGVYGNNGNNLPSASDAINLLG--- |
| → β-1,3-glucanase from Vitis riparia (67% homology) |
| II: TQFQLKNNTPFTKWPATLSGG--- |
| → thaumatin-like protein B from Arabidopsis thaliana (71% homology) |
| III:AQFNVVNRXPYTVWAAAVPGGGKQLNSGDSW--- |
| → thaumatin-like protein A from Vitis vinifera (71% homology) |
| IV:AQFNVVNRXPYTVWAAAVPGGGKQLNS--- |
| → thaumatin-like protein A from V. vinifera (70% homology) |
| V: RNVLVNNEGLYAGQSLVVEQY--- |
| → mannose-binding lectin from Allium fistulosum (100% homology) |
| VI: ELINLASMXYFGPMIQXDL--- |
| → 2S albumin precursor from Glycine max (79% homology) |
| VII:GARKWSGKVXALLPNSKPEQAWRLLKDFXNLHK--- |
| → lachrymatory factor synthase from A. fistulosum (93% homology) |
| VIII: GAXKWXGKVXALLPNXK--- |
| → lachrymatory factor synthase from A. fistulosum (73% homology) |
| IX: ATYNIYNPAQTNWDLNAVGA--- |
| → pathogenesis-related protein 4 from Lycoris radiata (90% homology) |
Secondly, major proteins contained in fractions 1–10 (Fig. 5) from hydrophobic interaction chromatography were subjected to SDS-PAGE, followed by electroblotting onto PVDF membranes and N-terminal sequencing. Fraction 2 gave a major band corresponding to band VI in Fig. 1. Since the band did not give an N-terminal amino acid sequence, fraction 2 was subjected to reversed-phase HPLC to isolate a 16 kDa protein on SDS-PAGE (Fig. 7A). Its internal amino acid sequence, which was obtained by analyzing a peptide fragment derived from Achromobacter protease I digestion of the CAM-16 kDa protein, was homologous to that of 2S albumin precursor from Glycine max (NP_001238443). Major protein bands observed with fractions 5–9 were identified as bands I-V in Fig. 1 (Fig. 5). These results showed that not only the lectin but also other proteins including TLPs enhanced the TNF-α production in RAW 264 cells.
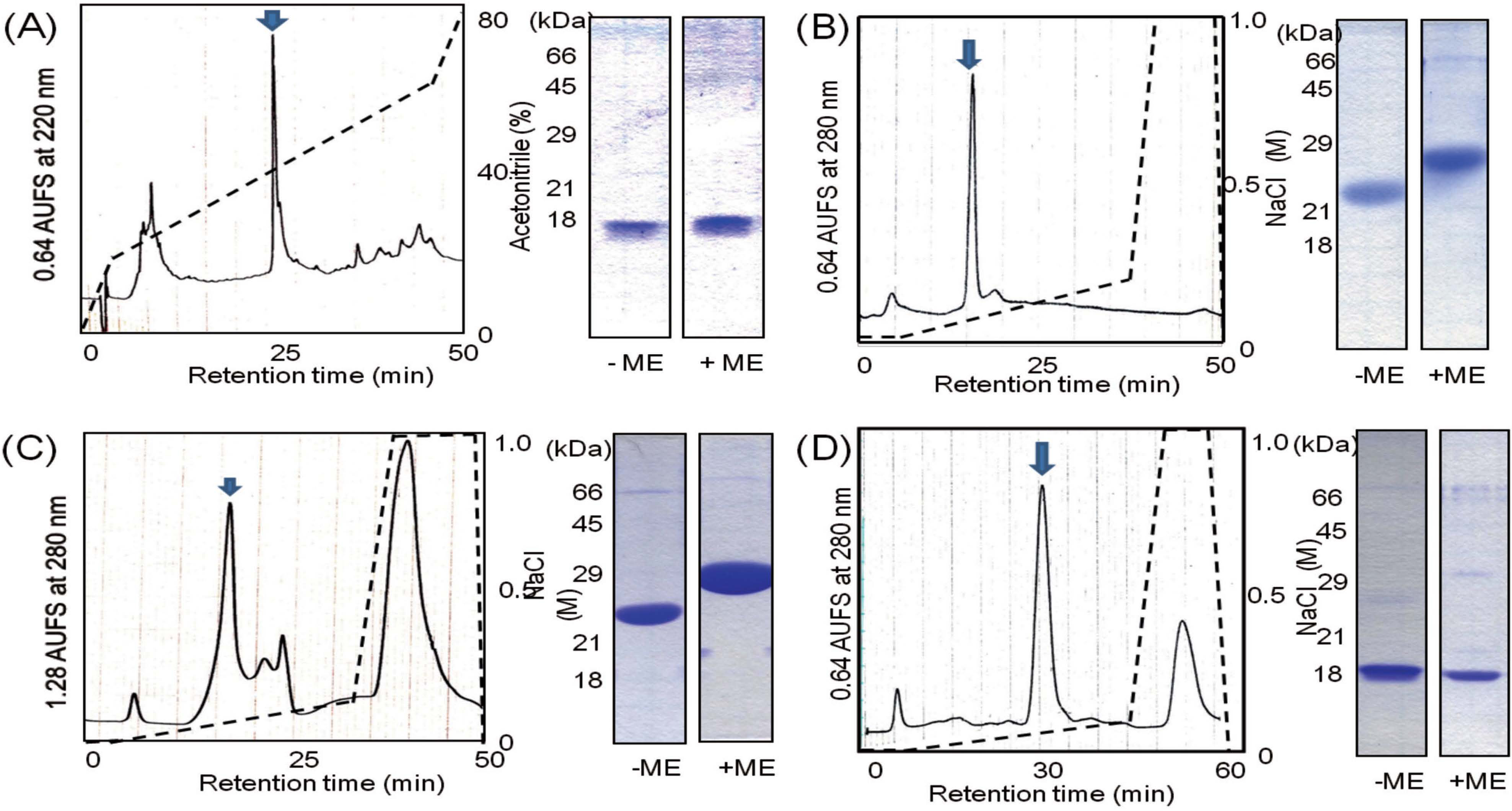
Isolation of mucus proteins. (A) 2S Albumin precursor was isolated by reversed-phase HPLC from fraction 2, obtained by hydrophobic interaction chromatography. Column: Wakosil 5C4-200 column. Fractions 5 (B), 8 (C), and 9 (D) were subjected to anion exchange chromatography on a HiTrap Q HP (5 mL) column pre-equilibrated with 50 mM Tris-HCl (pH 8.0/8.7), and eluted with a linear gradient of NaCl (0 – 1.0 M) in the same buffer at a flow rate of 1.0 mL/min. TLP A-a and TLP B, and mannose-binding lectin were isolated from each fraction, respectively. SDS-PAGE was performed on a 15% gel.
Isolation of TLPs and mannose-binding lectin To isolate TLP A-a, TLP B, and mannose-binding lectin, fractions 5, 8, and 9 were subjected to anion-exchange chromatography, respectively (Figs. 7B-D). TLP A-a was separated with a linear gradient of NaCl (0 to 1.0 M) in 50 mM Tris-HCl (pH 8.0). SDS-PAGE of TLP A-a on a 15% gel in the presence of ME showed a single major band at 26 kDa (Fig. 7B). On the other hand, TLP B purified under the same condition except for a linear gradient of NaCl in 50 mM Tris-HCl (pH 8.7) gave a single band at 28 kDa on SDS-PAGE (Fig. 7C). Both TLPs showed smaller apparent molecular masses in the absence of ME than in the presence of ME, indicating that TLPs formed intramolecular disulfide bonds. The mannose-binding lectin eluted with a linear gradient of NaCl in 50 mM Tris-HCl (pH 8.7) showed a single band at 18 kDa (Fig. 7D). Both TLPs and the lectin stimulated RAW 264 cells to produce TNF-α (Fig. 8).

TNF-α production in RAW 264 cells stimulated with TLP A-a, TLP B, and mannose-binding lectin isolated from the mucus protein fraction of Japanese bunching onion (Yoshikura cultivar). Data are presented as the mean ± SD of three experiments.
Comparison of mucus proteins of 13 cultivars Protein fractions were prepared from the green leaf mucus of 13 cultivars, Amarumeipponbuto, Shimonita, Jionji, Yoshikura, Omiyaguro, Nishida, Kiyotaki, Koshizuaigara, Kujobuto, Asagikeikujo, Akahige, Shokyu, and Fuyuwarabe, by (NH4)2SO4 precipitation (80% saturation). The yields of protein fractions were 0.4–0.8% (w/w) from the lyophilized mucus (Fig. 9). The protein fractions were separated by 15% SDS-PAGE and stained with CBB R-250 (Fig. 10). Major protein bands, which had been identified using the mucus protein of the Yoshikura cultivar, were detected in all cultivars. Their proximate protein compositions were analyzed by measuring the stained band intensities on the gels (Fig. 9). The composition ratios of VII (lachrymatory factor synthase a), I (β-1,3-glucanase), II (TLP B), III+IV (TLPs A-a and A-b), V (mannose-binding lectin), and VI (2S albumin precursor and PR4) were 12 – 20%, 6 – 10%, 13 – 23%, 2 – 14%, 6 – 10%, and 7 – 15%, respectively. These proteins occupied about 80% of total mucus proteins. The 26-kDa protein bands (TLP A-a) and the 18-kDa protein bands (mannose-binding lectin) of the 13 cultivars were subjected to N-terminal amino acid sequencing. Both of the N-terminal amino acid sequences were highly conserved (data not shown).

Yields and protein composition of the mucus proteins of 13 cultivars of bunching onion. Yields are presented as % (w/w) of the mucus protein fractions obtained by 80% saturated (NH4)2SO4 precipitation. Cultivars:① Amarumeipponbuto, ② Shimonita, ③ Jionji, ④ Yoshikura, ⑤ Omiyaguro, ⑥ Nishida, ⑦ Kiyotaki, ⑧ Koshizuaigara, ⑨ Kujobuto, ⑩ Asagikeikujo, ⑪ Akahige, ⑫ Shokyu, ⑬ Fuyuwarabe. I: β-1,3-glucanase, II: thaumatin-like protein B, III: thaumatin-like proteins A-a and A-b (IV), V: mannose-binding lectin, VI: 2S albumin precursor, VII: lachrymatory factor synthases a and b (VIII), X: other. Data are presented as the mean of three experiments.

SDS-PAGE patterns of the mucus proteins obtained by 80% saturated (NH4)2SO4 precipitation of 13 cultivars of Japanese bunching onion. ① Amarumeipponbuto, ② Shimonita, ③ Jionji, ④ Yoshikura, ⑤ Omiyaguro, ⑥ Nishida, ⑦ Kiyotaki, ⑧ Koshizuaigara, ⑨ Kujobuto, ⑩ Asagikeikujo, ⑪ Akahige, ⑫ Shokyu, ⑬ Fuyuwarabe. I: β-1,3-glucanase, II: thaumatin-like protein B, III: thaumatin-like protein A-a, IV: thaumatin-like protein A-b, V: mannose-binding lectin, VI: 2S albumin precursor, VII: lachrymatory factor synthase a, VIII: lachrymatory factor synthase b.
The mucus protein fractions of the 13 cultivars were assayed for TNF-α production in RAW 264 cells and hemagglutination against rabbit erythrocytes. The Fuyuwarabe cultivar (⑬) showed higher activities than other cultivars (Fig. 11), though the yield of the protein fraction was the lowest among the cultivars.

Activities of the mucus proteins of 13 cultivars of Japanese bunching onion. The protein samples were prepared under the conditions described in Fig. 9. The stimulatory activity of 50 µg/mL of each sample on TNF-α production in RAW 264 cells was measured. LPS (100 ng/mL) was used as a reference. Data are presented as the mean ± SD of three experiments. Lectin activities are presented as HA of each fraction at 1.0 mg/mL.
The green leaf mucus of Japanese bunching onion activated murine macrophages and splenic NK cells (Ueda et al., 2013). In addition, oral administration of the mucus to mice enhanced natural immunity, evidenced by the increased cytokine release and the phagocytic activity of macrophages. However, its active principles were not explored. The present study demonstrated that the protein fraction of the mucus was intimately related to the enhanced effect on TNF-α production in RAW 264 cells. Since some lectins of Allium sp. have been shown to enhance the production of TNF-α and IL-12 in RAW 264.7 cells and rat peritoneal macrophages (Prasanna and Venkatesh, 2015), and exhibit potent immunomodulatory activities in vitro and in vivo (Clement et al., 2010), we prepared protein fractions from Japanese bunching onion mucus by (NH4)2SO4 precipitation and examined their lectin activity using a hemagglutination assay. As expected, the protein fractions showed high lectin activity as well as an enhancing effect on TNF-α production in RAW 264 cells (Figs. 2 and 3).
The mucus protein fraction was further separated by hydrophobic interaction chromatography to confirm the presence of lectin molecules (Fig. 4). Most lectin activity could be detected in fraction 9, which contained a mannose-binding lectin (V) (Figs. 5 and 6). However, the enhancing effect on TNF-α production was observed not only in fraction 9 but also in other fractions (Fig. 6). These fractions contained various proteins, of which the N-terminal amino acid sequences were highly homologous to known bioactive proteins including TLPs, β-1,3-glucanase, lachrymatory factor synthase, and a pathogenesis-related protein (PR4). TLPs A-a and B and mannose-binding lectin were isolated from fractions 5, 8 and 9 by anion-exchange chromatography to confirm their effects on TNF-α production in RAW 264 cells. As shown in Fig. 8, it was confirmed that not only the lectin but also TLPs showed enhancing effects in a dose-dependent manner. We observed that both the lectin and TLPs have optimal concentrations to exhibit the enhancing effects; i.e., the lectin showed a lower optimal concentration than TLPs.
Lectins are a class of proteins that recognize carbohydrate structures and bind carbohydrates specifically. Lectins can be found in almost all organisms including plants, animals, fungi, bacteria and viruses. Plant lectins are classified into the following seven families based on their amino acid sequences: legume lectins, monocot mannose-binding lectins, jacalin-related lectins, Cucurbitaceae phloem lectins, chitin-binding lectins composed of hevein domains, amaranthin lectins, and ribosome-inactivating protein (Van Damme et al., 1998). The monocot mannose-binding lectins have been isolated from various species such as Amaryllidaceae, Alliaceae, Orchidaceae, and Liliaceae (Van Damme et al., 1993; 1998). The lectins have been extensively studied in terms of their insecticidal properties for a range of economically important pests (Ohizumi et al., 2009). Some plant lectins are stable against heating, changes in pH and digestive enzymes, and reach the intestinal tract by maintaining its structure and activity (Pusztai et al., 1995). For example, phytohemagglutinin administered to rats caused intestinal and systemic immune responses. It was also reported that garlic (A. sativum) lectin maintained its activity in the intestines and activated immunoreactivity when orally administered to mice (Clement et al., 2010). We have shown that lectins contained in foodstuff had modulating effects on the transport system of human intestinal Caco-2 cell monolayers (Ohno et al., 2006; Yamamoto et al., 2013; Nemoto et al., 2015). Thus, plant lectins can be considered as physiologically active ingredients in food resources.
Unlike animals, plants do not have acquired immunity because they lack a circulatory system and mobile immune cells. They self-protect by hardening the cell wall and producing antibiotic compounds like phytoalexins, tannins and pathogenesis-related (PR) proteins (Sels et al., 2008). PR proteins are defined as proteins that accumulate in plants upon pathological conditions or related situations. Currently, seventeen classes of PR proteins (PR1-17) are known in plants, and distinct sets of PR proteins are induced in response to different pathogens. TLPs share homologous sequences with thaumatin, a sweet-tasting protein isolated from the fruit of Thaumatococcus daniellii (Liu et al., 2010). Due to inducible expression by stressors like pathogens, TLPs are classified as PR5. Most TLPs have molecular masses ranging from 21 to 26 kDa and contain 16 conserved cysteine residues, which form intramolecular disulfide bonds and contribute high stability under thermal and pH conditions (Fierens et al., 2009). The high stability of TLPs agrees with the residual activity of the mucus protein after heat treatment (Fig. 3). This study showed that the bunching onion mucus contained at least three TLP molecules (bands II, III, and IV). The structural relation between TLP A-a (III) and TLP-A-b (IV), showing the same N-terminal amino acid sequences but different molecular masses, has not yet been revealed. There is accumulated evidence supporting the roles of TLPs in host defense and other physiological processes by transgenic over-expression, in vitro antifungal activity test, or related protein activity analyses (Liu et al., 2010). The average contents of TLPs and mannose-binding lectin were 26% and 8% in 13 cultivars of Japanese bunching onions, respectively (Fig. 9). These high protein contents may be related to the immune stimulatory activity of Japanese bunching onion mucus. It is probable that TLPs mediate signaling by interaction with their receptors on RAW 264 cells, as they have been shown to interact with various glycans and protein ligands (Liu et al., 2010). Although further studies are required to understand the mechanism, M cells are plausible targets for the orally administered mucus containing the lectin and TLPs. M cells express particular glycosylation patterns on their surfaces and transport a wide range of molecules from the intestinal lumen to the underlying lymphoid tissue, where a local and systemic potent immune response is initiated (Neutra et al., 1987). It has been reported that some plant lectins are able to induce T helper cell type 1 (Th1) cytokines upon interaction with glycosylated receptors on macrophages and/or dendritic cells, such as type 2 and 4 Toll-like receptors (TLR2 and TLR4), respectively (Souza et al., 2013). In addition, oral administration of a plant lectin to mice regulated Th2 immunity by exhibiting lower leukocyte trafficking and Th2 cytokine production.
There was no apparent relationship between the proximate compositions and the enhancing effects on TNF-α production of mucus proteins of the 13 cultivars (Figs. 9 and 11). Although a detailed explanation has not yet been obtained, it can be partly attributed to the fact that TLPs and lectin have different optimal concentrations to exhibit the activities in RAW 264 cells.
Besides these bioactive proteins, this study showed that other major protein components were potential bioactive proteins, such as lachrymatory factor synthase, PR4, β-1,3-glucanase, and 2S albumin precursor. This suggests that the protein components of Japanese bunching onion may play various roles in its food function described so far. Many cultivars of Japanese bunching onion are cultivated throughout Japan. Each cultivar has a characteristic commercial value due to the proportion of green leaves and white sheathes. For cultivars in which the white sheaths had a higher value, large quantities of the green leaves are discarded as agricultural waste. Thus, the green leaf mucus can be regarded as an underutilized resource of various bioactive proteins.
Acknowledgements We wish to thank Mr. Tadayuki Wako, senior researcher at the NARO Institute of Vegetable and Tea Science, for kindly providing the Japanese bunching onion samples. This work was supported by JSPS KAKENHI grant no. 26292111.