Abstract
The application of proanthocyanidins (PCs) in food industry is limited because of its instability and vulnerability against oxidative damage. In this work, by taking advantage of the reversible assembly characteristic of ferritin, apo-red bean ferritin (apoRBF)-PCs composites (FPs) were designed, and results showed a ratio of 1/10 of PCs (PCs/apoRBF, w/w) were encapsulated into a ferritin cage, and the encapsulation ratio was 23.8%. The FPs exhibited a homogeneous form, and maintained a spherical morphology. Further more, the thermal and light stability of PCs in FPs was significantly increased compared to free PCs (p < 0.05). The in vitro digestion indicated that apoRBF could prolong the release of PCs in simulated gastrointestinal tract. Additionally, the antioxidant activity of FPs was partly retained (56.6%) as compared to free PCs. This work offers a novel method to encapsulate and stabilize polyphenols and might be favorable for the application of ferritin in food industry.
Introduction
Natural proanthocyanidins (PCs), a major subgroup of flavonoids, are oligomers and polymers of polyhydroxy flavan-3-ol units (Deng et al., 2011). Its molecular distribution depends on the number of monomeric units that are linked to each other by C4 – C8 or C4 – C6 B-type bonds or doubly linked by a C4 – C8 bond and C2 – O7 A-type bonds (Ou and Gu, 2014). Several studies have confirmed that PCs exert various biological properties, such as antioxidant activity which are involved in the ability to scavenge superoxide radicals and hydroxyl radicals (Ioannone et al., 2015). Additionally, great attention have received in recent years due to their beneficial pharmacological effects such as anti-inflammatory, anti-allergy, anti-viral, anti-tumor activities (Li et al., 2002; Wang et al., 2014). However, the application in the food and pharmaceutical industries is limited primarily because of its basic structure of phenolic hydroxyl groups which can easily be affected by temperature, oxygen, and light exposes during processing and storage of the commodity. Moreover, PCs might be degraded to monomer and dimer forms and be low bioavailability during their transport in the stomach and intestinal tract (Li et al., 2015). Therefore, the development of novel technology to improve the stability and enhance its resistance to damage would be beneficial.
With the development of biotechnology and material science, many biological nanoparticles have shown great promise in bioactive molecular stabilization, delivery and disease diagnostics (Rica and Matsui, 2010; Zhang et al., 2014). Micro/nanoencapsulation of unstable bioactive compounds has attracted much attention in the food industry in various applications, such as the sustention of bioactivity and controlled release for improving bioavailability (Sari et al., 2015; Reza et al., 2008). The common techniques such as emulsification-solvent evaporation, emulsification-solvent diffusion, and precipitation methods are utilized to manufacture nano-sized particles (Bhawana et al., 2011; Horn and Rieger, 2001; Chen et al., 2014). However, these methods may have some disadvantages, easily resulting in non-uniform size of the particles, which may affect their sensory properties, storage, and bioavailability. In addition, these approaches usually employ considerable amounts of surfactants or organic solvents to prevent particle coalescence, resulting in sample contamination or environmental pollution. For these reasons, material selection is particularly important for preparing mono-dispersed and environmentally friendly nanoparticles.
Ferritin, a cage like protein, in nature offers a good opportunity to improve the stability of food bioactive molecules by encapsulation nanotechnology (Arosio et al., 2009; Zhao, 2010). Each ferritin consists of 24 identical/different subunits that self-assemble into a spherical cage-like structure (Fig. 1). The external diameter of the classical ferritin is about 12 – 13 nm, and the inner diameter is about 7 – 8 nm, and thus the inner chamber can accommodate up to ∼4500 iron atoms (Harrison and Arosio, 1996). Upon deprivation of the iron from the natural ferritin, the resulting apoferritin can provide a central cavity which can be efficiently loaded with transition metals, drugs, fluorescent molecules or contrast agents (Zhen et al., 2013; Sun et al., 2011). More importantly, each ferritin has a unique reversible assembly characteristic, that is, the ferritin cage can be disassociated into subunits at pH 2.0/11.0 or by addition of denaturants and then reconstituted when pH is adjusted to neutral condition or denaturant is removed (Kim et al., 2011; Liu et al., 2006). During this process, small molecules such as PCs can be added to the denatured lipid and thus be entrapped within the ferritin cage, thereby forming ferritin-small molecule nanoparticles.

The objective of this study was to fabricate homogeneous ferritin stabilized PCs nano-dispersions (FPs) using the reversible assembly property of apoferritin. The optimal encapsulation condition for FPs, particle morphology, photo and thermal stability, the in vitro release behavior of FPs in simulated digestive fluid, and the oxidative stability were evaluated.
Material and Methods
Chemicals and reagents Proanthocyanidins (98% purity) was purchased from Tianjin Jianfeng Natural Product R&D Co., Ltd. (Tianjin, China). Sodium dodecyl sulfate (SDS), Tris, TEMED, β-mercaptoethanol, Coomassie Bright Blue R-250, and electrophoresis marker were obtained from Solarbio Chemical Co. (Beijing, China). Pepsin and trypsin were purchased from Sigma Chemical Co. (Shanghai, China).
Isolation and purification of red bean (adzuki) seed ferritin Red bean (adzuki) ferritin and apo Red bean (adzuki) ferritin (apoRBF) was extracted and purification as previously described (Deng et al., 2010; Yang et al., 2014). The molecular weights of apoRBF were estimated by native PAGE using an 8% polyacrylamide gradient gel employing Tris-HCl (25 mM, pH 8.3) as running buffer, and the electrophoresis was run at 5 mA for 15 h, at 4°C. SDS-PAGE was applied to determine protein purity under denaturing conditions using 15% gels and the electrophoresis was run at 15 mA. Ferritin concentration was determined according to the Lowry method using BSA as a standard.
Preparation of FPs FPs was prepared by taking the advantage of the reversible assembly of ferritin (Kim et al., 2011). PCs were a class of polyphenol compounds that were stable under acidic condition and were easily degraded under alkaline conditions. Thus, in order to insure the stability of PCs, the preparing scheme is selected to operate under acid condition, and the scheme was shown in Fig. 2. PCs was dissolved in deionized water to make an aqueous solution with a final concentration of 7.0 mg/mL and stored in amber bottles at 4°C. Specifically, the pH value of apoRBF solution (5 mL, 0.56 g/mL) was adjusted to 2.0 by addition of HCl (1.0 M) to disassemble ferritin into subunits. Then the denatured apoRBF solution was stirred slowly for 30 min (20°C) under magnetic stirring. PCs aqueous solution was added to above apoRBF solution with the PCs/ apoRBF mass ratio (w/w) of 1/2, 1/5, 1/10, 1/15, 1/20, 1/ 25, 1/30, respectively. After 10 min, the pH of reaction solution was gradually increased to 6.8 with NaOH (1.0 M), and the resultant mixture solution was incubated at 4°C for 2 h in the dark to induce the reassembly of the ferritin cage. After this process, the PCs may be trapped inside of the ferritin cavity because the pore size of ferritin channels (0.3 nm in diameter) was less than the size of PCs molecule. The resulting mixture solution was then dialyzed (10 kDa cutoff) against Mops buffer (20.0 mM, 0.15 M NaCl, pH 6.8) four times at 6.0 h intervals, and the unbound PCs were removed from the mixture by diffusing out of the dialysis bag because the MW cut-off of membrane was much larger than that of the PCs. Finally, the suspension was filtered through 0.45-µm hydrophilic cellulose membrane filters, and a clarify FPs solution was obtained and then stored at 4°C.

On the other hand, ferritin-PCs mixture was prepared by simply mixing of PCs and apoRBF in a certain mass ratio without disassembly of the protein cage, followed by the same dialysis step to FPs. The PCs may associate with outer surface of protein shell and was used as control sample for further experiment.
Transmission Electron Microscopy (TEM) Analysis Protein and FPs solution (PCs/apoRBF = 1/10, w/w) samples can be diluted with 50 mM Mops buffer (pH 6.8) prior to being placed on carbon-coated copper grids. ApoRBF and FPs samples were negatively stained with 2% uranyl acetate for 5 min, and were imaged at 80 kV through a Hitachi H-7650 scanning electron microscope.
Dynamic light scattering (DLS) analyses DLS experiments were performed using a dynamic light scattering instrument (Malvern, UK), at 25°C. The samples with a final ferritin concentration of 0.4 mM were allowed to stand for 2 h prior to DLS measurement to ensure the reactions were complete.
Fluorescence spectra The fluorescence spectra of the PCs and FPs (PCs/apoRBF =1/10, w/w) samples were scanned using the RF-5301PC Spectrofluorophotometer (Shimadzu, Japan). The excitation wavelength was set at 290 nm, and emission wavelength was scanned from 290 nm to 500 nm at 25°C. Experiments were carried out in triplicate.
PCs encapsulation efficiency measurements The content of PCs was determined using the BuOH-HCl-FeIII method (Chen et al., 2016), and the absorbance of the samples were read at 550 nm using an 8453 UV-visible spectrophotometer (Agilent, USA). To determine the PCs content encapsulated in the ferritin cage, FPs samples were adjusted to pH 2.0 by addition of HCl (1 M) to disassemble the ferritin into subunits, resulting in the release of the PCs, followed by transferring into an Amicon Ultra-3K centrifugal filter device (Pall Corp.). After centrifugation at 8000 rpm for 20 min, PCs penetrated through the membrane into the centrifuge tube, which was determined by the PCs standard curve. The encapsulation ratio (%) was calculated according to following equation (1).
Thermal stability measurements The photo and thermal stability experiments were performed as previously reported with some modifications (Zhang et al., 2014). Briefly, to evaluate the stability of PCs encapsulated in ferritin exposed to UV radiation, 10.0 mL of FPs solution (0.56 mg/mL, PCs/apoRBF =1/10, w/w) were placed at a distance of 30 cm under an UV lamp (SW-CJ-1FD Series 20 W UV Lamps, Suzhou, China) with a wavelength of 254 nm for 48 h. Free PCs (0.056 mg/mL) was used as a control, and 0.6 mL of the solution was sampled at 2 to 48 h for PCs quantification. To evaluate the stability of PCs encapsulated in ferritin exposed to thermal processing, 10.0 mL of FPs solution (0.56 mg/mL, PCs/apoRBF =1/10, w/w) was set in a water bath (Model DK-8D, Tianjin Honour Instrument Co., Tianjin, China) at 37°C, and 50°C in a dark tube for 48h. The heated samples (0.4 mL) were collected separately after 2 to 48 h, for quantifying the amount of residual PCs as described above. PCs solution with identical amount of PCs content was used as a control sample for residual PCs determination. The residual ratio (%) of PCs was calculated according to equation (2) as following,
PCs release from FPs in simulated conditions Simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) digestion conditions were established as recently described (Zhao et al., 2011; Wada and Bo, 2014). Specifically, 40 mg of pepsin was dissolved in 2 mL HCl solution (1 mM) to prepare a 2% SGF, whereas SIF consists of 4 mg/mL trypsin buffer with potassium dihydrogen phosphate (8.00 g L−1), pancreatin (4.76 g L−1), and bile salts (5.16 g L−1) in the fluid. Firstly, pepsin-free SGF (2 g NaCl and 7 mL 32% HCl were added to 1 L distilled water) was placed in a heated water bath (37°C), FPs (0.56 mg/mL, PCs/apoRBF =1/10, w/w) and pepsin (2%) were added to the above simulated gastric fluid solution (8 mL) and the pH was adjusted to 2.0 by addition of HCl (1.0 M), and incubated in a water bath at 37°C. This gastric incubation was continuously stirred for 2.0 h, and lipid (0.2 mL) was collected at 0 to 120 min for released PCs quantification. Then, the pH of above digestion fluid was adjusted to 7.5 by addition of NaHCO3 (0.5 M), and 6.25 mL of SIF was added to above system to start the digestion reaction in the dark in a water bath at 37°C. After each 0 to 120 min, 0.3 mL of the FPs lipid were collected by a pipettor (Eppendoff), and were applied for centrifugation at 5000 rpm for 10 min. The obtained supernate was applied to PCs quantification. Free PCs were used as control sample for above procedures. The release ratio (%) of PCs was calculated according to equation (3) as following,
Antioxidant activity assay The DPPH radical-scavenging activity of the samples (0.02 mg/mL of free PCs; FPs with 0.2 mg/ mL of protein, an equivalent of 0.02 mg/mL of PCs; 0.2 mg/mL of apoRBF) was determined according to reported method (Kerdudo et al., 2014). An ethanol-water (80: 20) solution was used as control sample for detection; and the Tris-HCl buffer (50 mM, pH 6.8) were used as control samples for DPPH radical-scavenging activity detection. Absorbance at 517 nm was determined and antioxidant activity was calculated as followings:
Where Ao is the absorbance without sample and Ae is the absorbance with sample.
Statistical Analysis All experiments analyses were carried out in triplicate and all data were presented as mean ± standard deviation (SD). Statistical significance among treatments was determined using SPSS15.0 software. The analysis of variance was calculated at 5% level of significance.
Results and Discussion
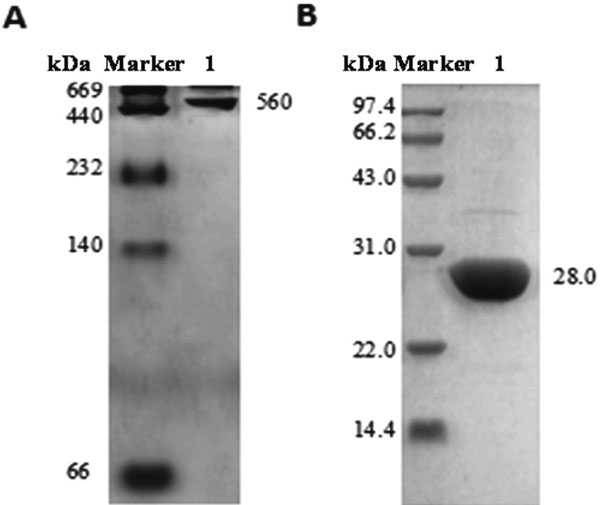
Characterization of apoRBF The molecular weight of apoRBF was determined by Native-PAGE and SDS-PAGE, and results are shown in Fig. 3. Native-PAGE showed that the purified apoBRF was a single complex, and the apparent molecular weight was determined to be about 560 kDa (Fig. 2A). SDS-PAGE indicated that apoBRF was composed of only one subunit with molecular weight of about 28.0 kDa (Fig. 2B), which was consistent with the reported literature (Li et al., 2013), indicating a successful preparation.
Preparation of FPs The primary parameter we concerned is the PCs encapsulation efficiency. The influence of the mass ratio of PCs/apoRBF on the encapsulation ratio of PCs was evaluated to obtain the best embedding effect. According to the equation (1), different adding mass ratios of 1/30, 1/25, 1/20, 1/15, 1/10, 1/5 and 1/2 (PCs/apoRBF) involved encapsulation ratios were compared (Fig. 4). It should be noted that upon continuing to increase PCs proportion (1/10 to 1/2), the encapsulation ratio did not increase accordingly, but even significantly decreased to 12.2% (P < 0.05), only a half value compared to the group with an adding ratio of 1/10 of PCs (PCs/apoRBF). PCs induced ferritin association might be a reason for the decrease of the encapsulation efficiency when PCs adding ratio increased. Obvious turbidity was happened as the PCs/apoRBF mass ratio increased to 1/5 (data not shown). The structure changes or the cross-linking between ferritins might inhibit the encapsulation efficiency.

Characterization of the FPs The dissolution state of three different samples (apoRBF, FPs, and free PCs solutions) was compared as shown in Fig. 5A. It was observed that both the FPs (PCs/apoRBF=1/10, w/w) and free PCs solutions were all transparent and showed good solubility in water, appearing the typical characteristic of the PCs (Fig. 5A, b and c). Thus, the water solubility and color of the PCs were not changed after the encapsulation. We believe that apoRBF-encapsulated PCs will not influence the color and good solubility of PCs in food application.

Subsequently, to obtain the evidence that PCs was successfully encapsulated within the apoRBF inner cavity, the morphology of FPs (PCs/apoRBF=1/10, w/w) was observed by TEM. Results showed a typical outside diameter of 12 nm in size for apoRBF (Fig. 5B). Similarly, the FPs also exhibited a homogeneous spherical structure with outside diameter of about 12 nm (Fig. 5C). However, there was an obvious distinction between the morphology of apoRBF and FPs, that is, black uranium cores were within most of the apoRBF cages (Fig. 5B, highlighted by arrow), whereas FPs not. It has been previously reported that if uranium flows into the ferritin cavity via channels, there would be black cores within the ferritin cage; however, if small molecules were successfully encapsulated in the protein cage, there would be no or small proportion of uranium-containing cores forming within the cavity because small molecules might occupy the inner cavity and prevent the entrance of uranyl acetate (Meldrum et al., 1991; Zhang et al., 2014). Thus, if the ferritin cavity was encapsulated with PCs molecules, there may be no or less black uranium cores forming within the cavity due to the prevention of the entrance of uranyl acetate into the ferritin. As expected, “white” nanoparticles with black uranium-containing cores disappeared within almost all FPs molecules (Fig. 5C). Additionally, TEM differences between ferritin-PCs mixture (simply mixed without reversible assembly of the ferritin, with black uranium cores) (Fig. 5D) and FPs (without black uranium cores) (Fig. 5C) could eliminate the possibility that the PCs only bound to the surface of the ferritin cage after disassembly. Thus, the PCs molecules were successfully encapsulated within apoRBF ferritin inner cavity, and the spherical morphology was not obviously changed after encapsulation.
The hydrodynamic radius (RH) of the apoRBF and FPs samples was further detected by DLS. The changes of relative intensity peak area represented by RH on size distribution can approximately reflect the changes in the relative amounts of different scattering objects (Yang et al., 2014). This result was in good accordance with the TEM image (Fig. 5E) that only one population (7.7 nm) was evident in the scattered light intensity distribution curve of apoRBF, whereas the FPs exhibited a similar RH of 8.0 nm but with a small portion of complexes at 29.3 nm, which might result from slight aggregation of FPs induced by PCs.
Stability of PCs in FPs against photo- and thermal-processing Phenolic compounds such as PCs are usually not chemically stable, and their bioavailability and biological half-life are usually significantly influenced when exposing to light, heat and oxidation (Cheynier et al., 1990; Liazid et al., 2007). To evaluate the protective function of the ferritin cage, the residual ratios of PCs in FPs during photo and thermal treatments were compared, and free PCs were used as a control sample. As for UV and natural light treatments (Fig. 6), it was observed that both PCs in FPs and free PCs undergone different extent of degradation during storage. Briefly, a rapid degradation of free PCs was observed with residual ratios of only 42.67% after UV treatment over 48 h, which was significantly lower than that of FPs (57.73%) (P < 0.05) (Fig. 6A). Similarly, upon natural light processing, the residual ratios of PCs in FPs (67.73%) was significantly higher than that of free PCs (52.67%) in 48 h (P < 0.05) (Fig. 6B). On the other hand, after incubation at 37°C for 48 h, the residual ratio of free PCs was decreased to 55.67%, however, this value was significantly lower than that of FPs (75.25%) (P < 0.05). The 50°C treatment also displayed a similar trend of residual ratios for free PCs and PCs in FPs (Fig. 6D). It should be noted that higher temperature treatment (50°C) could highlight the protective function of the ferritin cage, because the PCs in FPs showed a more mitigative degradation trend, rather than a rapid degradation of free PCs. These results indicated that the PCs degradation in FPs was reduced, and the ferritin cage showed a protective function to improve the stability of PCs upon photo- and thermal-processing.

It has been reported that PCs was sensitive to surrounded factors such as metal ions, oxidizing agent, light, and thermal treatment, resulting in the degradation and lose of bioactive functions (Liazid et al., 2007; Zou et al., 2012). To encapsulate PCs and thus inhibit its degradation by certain methods is necessary. In this work, the primary reasons of the protective effect of ferritin for PCs may be manifested in the following aspects. Firstly, it has been revealed that spherical ferritin shell was stable and kept intact against thermal treatment even at 80°C for 10 min, indicative of a high thermal stability (Stefanini et al., 1996), thus, the ferritin can act as a physical barrier which would effectively insulate the PCs from external temperature changes in food processing system. Another reason may lie in the ferritin-PCs interaction, such as hydrophobic interactions or van der Waals, these weak forces may contribute to the resistance from photo- and thermal-degradation (Qian et al., 2012). Therefore, ferritin as a natural nanocarrier is potential for the stabilization of bioactive molecules in food industry.
PCs release from FPs in gastrointestinal tract The release behavior may influence the absorption and bioavailability of one targeted polyphenol in gastrointestinal digestion (Pei et al., 2012). Reports have shown that the polyphenols are susceptible to digestive liquid substances (such as pepsin and trypsin), which may lead to structural damage or degradation (Tenore et al., 2015). Decorating of the PCs with the ferritin cage might influence its release in the gastrointestinal tract. In this work, the release properties of PCs in simulated gastrointestinal tract were evaluated and free PCs were used as a control sample. As shown in Fig. 7A, free PCs displayed a burst release within 20 min in simulated gastric fluid and the release ratio reached to 50.2%, after 120 min of incubation the release ratio was up to 86.1%. In contrast, PCs release from FPs showed a more moderate process, and the release ratio of PCs in FPs was lower after encapsulation by ferritin with release rate of 66.2% after 120 min. The reason why ferritin cage could slow down the release ratio of the PCs in the same time range might due to the slow degradation of ferritin induced by pepsin. Thus, along with the degradation of the ferritin shell, the PCs encapsulated by ferritin might gradually leak from the cage and displayed a sustained release behavior as compared with the free PCs.

In simulated intestinal fluid (Fig. 7B), free PCs independent of ferritin embedding also exhibited a rapid release process with a 58.1% release ratio within 30 min, followed by a continuous release up to 86.9% after another 1 h. Contrastively, PCs in FPs showed a relative slower and sustained release process under simulated intestinal conditions, and the release ratios reached around 68.7% after 2 h, respectively. Combined with the above simulated results in simulated gastric fluid, it can be deduced that ferritin cage exhibited more pronounced effect to prolong the release of the PCs, and this effect would be beneficial to the minimization of PCs release, and may also be meaningful to improve the bioavailability of PCs during their transport in the stomach and intestinal tract in human digestive system (Li et al., 2015). It should be noted that polyphenols will continuously pass through the gastric and intestinal tract, and thus be absorbed by intestine or excreted from the body. In vitro gastric digestion might affect the release and absorption of the PCs on intestinal digestion. Therefore, to obtain the relative veritable results of PCs release behavior, the PCs release in vitro from FPs was performed in a continuous manner to pass the simulated gastric and intestinal digestion in the present work.
Antioxidant property of FPs It has been shown that PCs has the ability to scavenge superoxide radicals and hydroxyl radicals, exhibiting high antioxidant activity (Ioannone et al., 2015). After PCs was encapsulated within the apoRBF, the antioxidant activity of PCs in FPs was noteworthy investigated. Fig. 8 showed a comparison of the antioxidant activities of free PCs, apoRBF, and FPs (PCs/apoRBF=1/10, w/w) as measured by the DPPH scavenging capacity assay. It can be found that the radical-scavenging abilities of the three samples were dependent on the concentration in a range of 0.02 – 0.04 mg/mL. Results also showed that the DPPH scavenging capability of apoRBF (4.1%, 0.02 mg/mL) was the lowest among the three samples, indicating that the ferritin was lack of antioxidant activity. By contrast, free PCs exhibited the DPPH scavenging capability of 69.2% (0.02 mg/mL), which was significantly higher than that of FPs (49.6%, 0.02 mg/mL). Similar results were also obtained when the assay was performed at higher sample concentrations (0.03 and 0.04 mg/ mL). Thus, although the PCs molecules were separated by ferritin shell (2 nm) from the solution, lower DPPH scavenging capability of FPs was also existed, and the ferritin could retain part of the antioxidant activity (56.6%, 0.02 mg/mL) of PCs.

Several reports have proved that the hydrogen-donating ability of the compound closely contributes to its DPPH radical scavenging ability (Yang et al., 2008; Chat et al., 2011). After encapsulation in apoRBF, PCs molecules could be physically separated from the external environment by the protein shell (2 nm in thickness). However, this shielding effect of the ferritin cage did not absolutely counteract the DPPH radical-scavenging ability of PCs in FPs, indicating that the hydrogen-donating capacity of FPs was changed as a result of the ferritin-PCs interaction. As compared with the apoRBF, the significantly higher values of DPPH radical-scavenging ability for ferritin-PCs complexes might result from the hydrogen bonds that were formed between hydrogen atoms in the hydroxyl groups of PCs with the electro-negative atoms of interior surface of ferritin. Upon the formation of the FPs, the hydrogen bonds between PCs and apoRBF may weaken the covalent bonds between hydrogen and oxygen in the hydroxyl groups, which in turn may facilitate the hydrogen donation by the hydroxyl groups of PCs (Nguyen et al., 2013). In spite of this, it must be noted that 2 nm thickness of the ferritin can weaken the improved hydrogen donation ability of PCs in FPs, resulting in the relative lower DPPH radical scavenging ability of FPs as compared to free PCs.
Conclusion
In this study, by using the reversible assembly characteristics of ferritin, homogenous dispersion of FPs were fabricated. Results indicated that proanthocyanidins were successfully encapsulated into the ferritin cage, and the encapsulation ratio was up to 23.2%. Compared to the free PCs, the light and thermal stability of the PCs in the FPs was significantly increased. In addition, the in vitro digestion indicated that the ferritin cage could prolong the release of PCs in the gastrointestinal tract. Further more, the antioxidant activity of FPs was still retained but was lower as compared to that of free PCs molecules. We believe that ferritin as a natural nanocarrier is beneficial for the stabilization of bioactive molecules. This work provides a novel method to encapsulate food resource active ingredient and is worthy of further investigation with great potential.
Acknowledgement This work was financially supported by Nature Science Foundation of China (No. 31501489, 31501544), Nature Science Foundation of Tianjin (youth program) (16JCQNJC14500), and Nature Science Foundation of China (31471701).
References
- Arosio, P., Ingrassia, R., and Cavadini, P. (2009). Ferritins: a family of molecules for iron storage, antioxidation and more. BBA-Biomembranes, 1790, 589-599.
- Bhawana Basniwal, R. K., Buttar, H. S., Jain, V. K., and Jain, N. (2011). Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J. Agr. Food Chem., 59, 2056-2061.
- Chat, O. A., Najar, M. H., Mir, M. A., Rather, G. M., and Dar, A. A. (2011). Effects of surfactant micelles on solubilization and dpph radical scavenging activity of rutin. J. Colloid. Interf. Sci., 355, 140-149.
- Chen, L., Bai, G., Yang, S., Yang, R., Zhao, G., and Xu, C. (2014). Encapsulation of curcumin in recombinant human h-chain ferritin increases its water-solubility and stability. Food Res. Int., 62, 1147-1153.
- Chen, M. H., Mcclung, A. M., and Bergman, C. J. (2016). Concentrations of oligomers and polymers of proanthocyanidins in red and purple rice bran and their relationships to total phenolics, flavonoids, antioxidant capacity and whole grain color. Food Chem., 208, 279-287.
- Cheynier, V., Rigaud, J., Souquet, J. M., Duprat, F., and Moutounet, M. (1990). Must browning in relation to the behavior of phenolic compounds during oxidation. Am. J. Enol. Viticult., 41, 346-349.
- Deng, J., Cheng, J., Liao, X., Zhang, T., Leng, X., and Zhao, G. (2010). Comparative study on iron release from soybean (Glycine max) seed ferritin induced by anthocyanins and ascorbate. J. Agr. Food Chem., 58, 635-641.
- Deng, J., Li, M., Zhang, T., Chen, B., Leng, X., and Zhao, G. (2011). Binding of proanthocyanidins to soybean (glycine max) seed ferritin inhibiting protein degradation by protease in vitro. Food Res. Int., 44, 33-38.
- Harrison, P. M. and Arosio, P. (1996). The ferritins: molecular properties, iron storage function and cellular regulation. Biochimica. Et. Biophysica. Acta., 1275, 161-203.
- Horn, D. and Rieger, J. (2001). Organic nanoparticles in the aqueous phase—theory, experiment, and use. Angew. Chem. Int. Edit., 40, 4330-4361.
- Ioannone, F., Mattia, C. D. D., Gregorio, M. D., Sergi, M., Serafini, M., and Sacchetti, G. (2015). Flavanols, proanthocyanidins and antioxidant activity changes during cocoa (theobroma cacao, l.) roasting as affected by temperature and time of processing. Food Chem., 174, 256-262.
- Kerdudo, A., Dingas, A., Fernandez, X., and Faure, C. (2014). Encapsulation of rutin and naringenin in multilamellar vesicles for optimum antioxidant activity. Food Chem., 159, 12-19.
- Kim, M., Rho, Y., and Sikjin, K. (2011). Ph-dependent structures of ferritin and apoferritin in solution: disassembly and reassembly. Biomacromolecules, 12, 1629-1640.
- Li, M., Yun, S., Yang, X., and Zhao, G. (2013). Stability and iron oxidation properties of a novel homopolymeric plant ferritin from adzuki bean seeds: a comparative analysis with recombinant soybean seed h-1 chain ferritin. Biochimica. Et. Biophysica. Acta., 1830, 2946-2953.
- Li, Q., Chen, J., Li, T., Liu, C., Wang, X., and Dai, T. (2015). Impact of in vitro simulated digestion on the potential health benefits of proanthocyanidins from choerospondias axillaris, peels. Food Res. Int., 8, 378-387.
- Li, WG., Zhang, X. Y., Wu, Y. J., and Tian, X. (2002). Anti-inflammatory effect and mechanism of proanthocyanidins from grape seeds. Acta. Pharmacol. Sin., 22, 1117-1120.
- Liazid, A., Palma, M., Brigui, J., and Barroso, C. G. (2007). Investigation on phenolic compounds stability during microwave-assisted extraction. J. Chromatogr. A., 1140, 29-34.
- Liu, G., Wang, J., Lea, S. A., and Lin, Y. (2006). Bioassay labels based on apoferritin nanovehicles. Chembiochem., 7, 1315-1319.
- Meldrum, F. C., Wade, V. J., Nimmo, D. L., Heywood, B. R., and Mann, S. (1991). Synthesis of inorganic nanophase materials in supramolecular proteincages. Nature, 349, 684-687.
- Nguyen, T. A., Liu, B., Zhao, J., Thomas, D. S., and Hook, J. M. (2013). An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem., 136, 186-192.
- Ou, K. and Gu, L. (2014). Absorption and metabolism of proanthocyanidins. J. Funct. Foods., 7, 43-53.
- Pei, S. L., Sun, G. Y., Choi, Y., Ha, T. V. A., and Ko, S. (2012). Physiochemical properties and prolonged release behaviours of chitosan-denatured β-lactoglobulin microcapsules for potential food applications. Food Chem., 134, 992-998.
- Qian, C., Decker, E. A., Xiao, H., and Mcclements, D. J. (2012). Physical and chemical stability of β-carotene-enriched nanoemulsions: influence of ph, ionic strength, temperature, and emulsifier type. Food Chem., 132, 1221-1229.
- Reza Mozafari, M., Kianoush Khosravi-Darani, G., Borazan, G., Cui, J., Pardakhty, A., and Yurdugul, S. (2008). Encapsulation of food ingredients using nanoliposome technology. Int. J. Food Prop., 11, 833-844.
- Rica, R. D. L. and Matsui, H. (2010). Cheminform abstract: applications of peptide and protein-based materials in bionanotechnology. Chem. Soc. Rev., 39, 3499-3509.
- Sari, T. P., Mann, B., Kumar, R., Singh, R. R. B., Sharma, R., and Bhardwaj, M. (2015). Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocolloid., 43, 540-546.
- Stefanini, S, Cavallo, S., Wang, C. Q., Tataseo, P., Vecchini, P., and Giartosio, A. (1996). Thermal stability of horse spleen apoferritin and human recombinant h apoferritin. Arch. Biochem. Biophys., 325, 58-64.
- Sun, C., Yang, H., Yuan, Y., Tian, X., Wang, L., and Guo, Y. (2011). Controlling assembly of paired gold clusters within apoferritin nanoreactor for in vivo kidney targeting and biomedical imaging. J. Ame. Chem. Soc., 133, 8617-8624.
- Tenore, G. C., Campiglia, P., Giannetti, D., and Novellino, E. (2015). Simulated gastrointestinal digestion, intestinal permeation and plasma protein interaction of white, green, and black tea polyphenols. Food Chem., 169, 320-326.
- Wada, Y. and Bo, L. (2014). Effects of industrial heating processes of milk-based enteral formulas on site-specific protein modifications and their relationship to in vitro and in vivo protein digestibility. J. Agr. Food Chem., 62, 6787-6798.
- Wang, S., Dong, S., Zhang, R., Shao, H., and Liu, Y. (2014). Effects of proanthocyanidins on porcine pancreatic lipase: conformation, activity, kinetics and thermodynamics. Process. Biochem., 49, 237-243.
- Yang, J., Guo, J., and Yuan, J. (2008). In vitro antioxidant properties of rutin. Lebensmittel-Wissenschaft und-Technologie, 41, 1060-1066.
- Yang, R., Chen, L., Zhang, T., Yang, S., Leng, X., and Zhao, G. (2014). Self-assembly of ferritin nanocages into linear chains induced by poly (β, l-lysine). Chemical Communications: cambridge, England., 50, 481-483.
- Zhang, T., Lv, C., Chen, L., Bai, G., Zhao, G., and Xu, C. (2014). Encapsulation of anthocyanin molecules within a ferritin nanocage increases their stability and cell uptake efficiency. Food Res. Int., 62, 183-192.
- Zhao, G. (2010). Phytoferritin and its implications for human health and nutrition. BBA-Gen. Subjects., 1800, 815-823.
- Zhao, L. P., Xiong, H., Peng, H., Wang, Q., Han, D., and Bai, C. Q. (2011). Peg-coated lyophilized proliposomes: preparation, characterizations and in vitro release evaluation of vitamin e. Eur. Food Res. Tech., 232, 647-654.
- Zhen, Z., Tang, W., Guo, C., Chen, H., Lin, X., and Liu, G. (2013). Ferritin nanocages to encapsulate and deliver photosensitizers for efficient photodynamic therapy against cancer. Acs. Nano., 7, 6988-6996.
- Zou, T., Li, Z., Percival, S. S., Bonard, S., and Gu, L. (2012). Fabrication, characterization, and cytotoxicity evaluation of cranberry procyanidins-zein nanoparticles. Food Hydrocolloid., 27, 293-300.












