2023 年 29 巻 1 号 p. 35-45
2023 年 29 巻 1 号 p. 35-45
The aim of this study was to investigate the protective effect of polyphenol extract from Highland barley (HBPE) on cisplatin (CDDP)-induced oxidative damage and mitochondrial damage in rat kidney. The experimental rats were divided into control group, CDDP group, HBPE (600 mg/kg) group, CDDP+HBPE (150 mg/kg) group, CDDP+HBPE (300 mg/kg) group and CDDP+HBPE (600 mg/kg) group. Rats in each group were intragastrically fed with normal saline and corresponding dose of HBPE for 21 d. On the 16th day, CDDP group and CDDP+HBPE group were injected intraperitoneally with CDDP (7.5 mg/kg), and the other groups were injected with the same amount of normal saline. On the 22nd day, the rats were killed and blood samples were taken to detect the contents of blood urea nitrogen (BUN) and creatinine (CrE) in serum, and calculate the kidney index. The kidney samples were used for the measurement of malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), lactate dehydrogenase (LDH), malate dehydrogenase (MDH), hexokinase (HK), and processed for histopathological examinations. The results showed that HBPE improved the abnormalities of serum CrE, BUN, antioxidant and carbohydrate metabolic enzymes in kidney induced by CDDP. HBPE can also reduce the degeneration of renal tubules and glomerular atrophy. Therefore, HBPE has a significant protective effect on oxidative stress injury and mitochondrial damage induced by CDDP in rats.
Cisplatin (CDDP) is a widely used anticancer drug in clinic (Dasari and Tchounwou, 2014; Galgamuwa et al., 2016). In the current clinical application of combined chemotherapy regimens, with CDDP as the main drug or with the participation of CDDP accounted for 70%–80%, and CDDP has achieved significant results in the treatment of a variety of malignant tumors (Ghosh, 2019; Makovec, 2019). However, CDDP will cause many toxic and side effects to human body in the process of treatment, among which nephrotoxicity is the most serious (El-Gizawy et al., 2020; Manohar and Leung, 2018). Studies have shown that the incidence of nephrotoxicity and the degree of kidney damage caused by cisplatin is proportional to the dose of cisplatin, and its manifestations include reversible acute renal injury and irreversible chronic renal failure (Song et al., 2020). And studies have shown that oxidative stress is the main cause of renal toxicity induced by CDDP (Chen et al., 2019; Yang et al., 2019). Relevant studies have shown that CDDP can destroy the metabolic balance of oxygen free radicals in vivo and cause oxidative stress (Li et al., 2020; Wang et al., 2020). Oxidative damage related to oxidative stress may be the main cause of CDDP induced renal toxicity, and mitochondrial dysfunction is the central link of CDDP induced renal toxicity.
Highland barley is a kind of barley, also known as naked barley, which belongs to Gramineae barley and belongs to the variety of cultivated barley in botany (Zhang et al., 2021a). It is called naked barley because its grain is separated from caryopsis (Obadi et al., 2021). Highland barley grows in the Qinghai-Tibet Plateau, which is about 1 400 meters to more than 4 700 meters above sea level (Zhao et al., 2020). It is the largest crop in Tibetan areas and the main food rations for farmers and herdsmen in Tibetan areas (Zheng et al., 2020). Phenolic compounds such as flavonoids, phenolic acids, diterpenes, and tannins have received attention for their high antioxidative activity (Kim and Lee, 2004). A large number of studies have shown that highland barley contains a large number of polyphenols, which has obvious antioxidant, anti-tumor, hypolipidemic and hypoglycemic effects (Ge et al., 2021; Liu et al., 2018; Shen et al., 2016; Zhang et al., 2021b). In the early stage, some researchers extracted large amounts of polyphenols from Highland barley with 80% acetone (Ge et al., 2021). Polyphenol extract from Highland barley (HBPE) is expected to be developed as a functional food to inhibit renal toxicity of CDDP and enhance its chemotherapy effect and anti-tumor activity.
In this study, CDDP was used to induce nephrotoxicity in rats and HBPE was given. The changes of serum urea nitrogen (BUN) and creatinine (CrE) were observed, and the antioxidant indexes such as contents of glutathione (GSH) and malondialdehyde (MDA), and activity of superoxide dismutase (SOD) in rat renal cortex were detected. Carbohydrate marker metabolic enzymes: lactate dehydrogenase (LDH), malate dehydrogenase (MDH), hexokinase (HK) activities, and renal pathological sections were observed to explore the mechanism of mitochondrial damage in CDDP nephrotoxicity and the protective effect of HBPE on oxidative stress injury and renal mitochondrial damage induced by CDDP.
Chemical and drugs Powder injection of CDDP was purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). HBPE was obtained from the Institute of Quality Standards & Testing Technology for Agro-Products, Chinese Academy of Agricultural Sciences (Beijing, China). HBPE has been qualitatively and quantitatively analyzed by the institute, and the Composition And Content Of Phenolic Compounds In Hbpe are shown in Table 1. All assay kits for BUN, CrE, GSH, MDA, SOD, HK, MDH, and LDH were acquired from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The Folin-Ciocalteu reagent was purchased from Beijing Solarbio Science & Technology Co. Ltd. (Beijing, China). All other chemicals and solvents were of analytical grade.
| Name | Free polyphenols (mg/kg DW) |
Bound polyphenols (mg/kg DW) |
Total (mg/kg DW) |
|---|---|---|---|
| quercetin | 0.06 | 0.05 | 0.11 |
| rutin | 0.614 | 0.388 | 1.002 |
| p-coumaric acid | 0.152 | 6.011 | 6.163 |
| protocatechuic acid | 0.159 | 0.48 | 0.639 |
| erucic acid | 0.159 | 0.48 | 0.639 |
| epicatechin | 9.04 | 0.558 | 9.598 |
| resveratrol | 0.041 | 0.031 | 0.072 |
| catechin | 7.791 | 0.545 | 8.336 |
| isorhamnetin | 0.061 | 0.038 | 0.099 |
| apigenin glycoside | 0.03 | 0.018 | 0.048 |
| vanillic acid | 0.537 | 2.799 | 3.336 |
| syringic acid | 0.214 | 0.82 | 1.034 |
| gallic acid | 1.455 | 3.771 | 5.226 |
| catechol | 0.118 | 0.099 | 0.217 |
| chlorogenic acid | 0.017 | 0.019 | 0.036 |
| caffeic acid | 0.242 | 4.43 | 4.672 |
| ferulic acid | 0.888 | 94.809 | 95.697 |
Animals This study is based on our institutional guidelines for the study of live animals, and the experimental scheme is approved by the Experimental Animal Ethics Committee of the functional Food functional Test Center of the School of Arts and Sciences of Beijing Union University. Sixty adult male SD rats, weighing 160 ± 20 g, were purchased from Beijing Hfk Bioscience Co.,Ltd (Beijing, China) and were housed under standard laboratory conditions (12 h light, 12 h dark and 24 ± 3 °C). The maintenance feed was produced by Beijing Keao Xieli Feed Co.,Ltd. All groups were given maintenance feed and rats were weighed every 3 d.
Determination of total phenol content Phenolic content was determined in terms of Folin-Ciocalteu colorimetric method described by Payet et al. with minor modifications (Payet et al., 2006). The 0.5 mL diluted extracts or standard solutions was mixed with 2.5 mL deionized water and 0.5 mL 1.0 M Folin-Ciocalteu reagent in volumetric flask. After 10 min, 1.5 mL 7.5% sodium carbonate was added and mixed thoroughly. After 1 h of reaction at room temperature, the absorbance was read at 760 nm. Total phenolics were expressed as mg gallic acid equivalents (GAE)/100 g DW.
HPLC determination of phenolic compounds The system used by HPLC is Nexera LC (Shimadzu, Kyoto, Japan). HPLC analyses were performed using a Dual gradient pump equipped with a ACQVITY UPLC BEH C18 column (50 × 2.1 mm, 1.7 µm). Elution was carried out with containing solvent A (0.1% formic acid in high purity water) and solvent B (methanol) as a mobile phase. The specific gradient procedure was as follows: 0–12 min, 5% B; 12–17 min, 30% B; 17–22 min, 60% B; 22–35 min, 5% B. The solvent flow rate was 0.15 mL/min at room temperature, and the injection volume was 20 µL. The phenolic extracts and standard compounds were analyzed under the same analysis conditions, and all of the above experiments were replicated three times. Identification of the main phenolic compounds was performed by comparisons to the retention time and UV spectra of authentic standards. The concentrations of phenolic compounds were calculated according to standard curves, and the results for the main phenolic compounds were expressed in micrograms per gram of dry weight (mg/kg DW).
Experimental design and drug administration SD rats were fed adaptively for one week and randomly divided into six groups with 10 rats in each group. They were control group, CDDP group, HBPE (600 mg/kg) group, CDDP + HBPE (150 mg/kg) group, CDDP + HBPE (300 mg/kg) group and CDDP + HBPE (600 mg/kg) group. CDDP + HBPE group and HBPE group were given corresponding doses of HBPE solution daily for continuous gavage of 21 d. Control group and CDDP group were given equal amount of distilled water for 21 d. 16 d after rats were given intragastric administration, the CDDP group and the CDDP+HBPE group were intraperitoneally injected with CDDP (7.5 mg/kg), which is known to cause nephrotoxicity. Control group and HBPE group were intraperitoneally injected with the same amount of normal saline.
Sample Collection On the 21st day of the experiment, the rats were fasted at night, and on the 22nd day, the blood was taken from the femoral artery and killed, and the blood was collected into a non-anticoagulant tube. After the blood was coagulated, the blood was centrifuged by 10 min (3 000 r/min, 4 °C). The upper serum was absorbed and the levels of BUN and CrE in rat serum were detected by automatic biochemical analyzer (Weitu Science Company of the Netherlands). Under ice bath conditions, the bilateral kidneys were quickly removed, the renal capsule was removed, weighed, and the kidney index was calculated using the following formula. One side of the renal cortex was quickly put into a liquid nitrogen tank and then quickly transferred to the refrigerator at −80 °C for subsequent biochemical detection of the renal cortex. The other kidney was soaked in 10% formalin and fixed for more than seven days for pathological sections.
 |
Preparation of 10% renal cortical homogenate The kidney cortex (0.2–1 g) was taken and rinsed with ice-cold saline, wiped dry with filter paper, accurately weighed and placed in an ice-bath beaker. Pre-chilled saline was added at a ratio of 1:9, and the homogenate was ground with a tissue grinder until there was no granular tissue visible to the naked eye. The prepared homogenate was centrifuged at 3 000 r/min for 10–15 min at 4°C, and the supernatant was taken for subsequent assays.
Determination of oxidative stress markers The contents of GSH, MDA and activities of SOD, LDH, MDH and HK in kidney of rats were detected by kit.
Histopathological examination Kidney specimens fixed in 10% formalin (v/v) were treated (dehydrated in graded concentrations of alcohol, soaked in xylene) and paraffin embedded. The slices were cut with 5µm thickness on a rotary slicer and stained with hematoxylin and eosin (H&E) after installation. Sections were evaluated under light microscopy.
Statistical analysis The experimental results were expressed as mean ± standard deviation (X ± sd), SPSS 12.0 statistical software was used for statistical analysis, and oneway ANOVA was used to deal with differences between groups. p < 0.05 was considered as significant difference.
Total phenol content and phenol compounds determination of Highland barley The total phenolic content of HBPE provided by the Institute of Quality Standards & Testing Technology for Agro-Products, Chinese Academy of Agricultural Sciences was determined by Folin-Ciocalteu colorimetric method and the result was 450 mg ± 5.0 mg GAE/100 g DW. And the results of HPLC determination of phenolic compounds in highland barley are shown in Table 1.
Observation of general index The changes of body weight of rats in each group with the number of feeding days are shown in Fig. 1. Rats in control group and HBPE group grew well, their hair was smooth and bright, their activity was quick, and their body weight increased steadily. After injection of CDDP, with the increase of feeding days, the rats in the CDDP group gradually decreased their body weight and showed symptoms such as lethargy, lethargy, back curling, hair loss, and abnormal stools. Compared with the CDDP group, the CDDP+HBPE group also showed the above abnormalities, but to a lesser extent. As shown in Table 2, CDDP significantly increased the kidney index of rats (p < 0.01), while there was no significant difference between the kidney index of HBPE (600 mg/kg) group and normal control group. the kidney index of HBPE+CDDP (600 mg/kg) group was significantly lower than that of CDDP model group (p < 0.01).
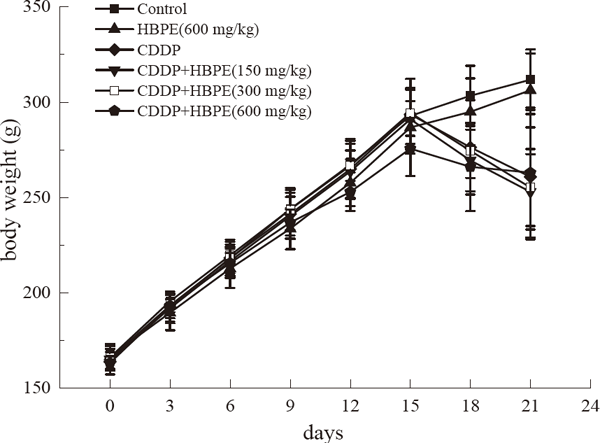
The changes of body weight of rats in each group with feeding days. (n = 10).
| Groups | Kidney index (%) |
|---|---|
| Control | 0.72 ± 0.09 |
| HBPE (600 mg/kg) | 0.83 ± 0.11 |
| CDDP | 1.10 ± 0.12** |
| HBPE (150 mg/kg) + CDDP | 1.13 ± 0.08 |
| HBPE (300 mg/kg) + CDDP | 1.04 ± 0.14 |
| HBPE (600 mg/kg) + CDDP | 0.87 ± 0.17## |
Data are expressed as means ± SEM. n = 10 for each experimental group.
Nephrotoxic markers on HBPE treatment The results of serum markers for renal injury are shown in Fig. 2. Compared with the control group, cisplatin significantly increased the levels of BUN and CrE in serum of rats, suggesting that cisplatin caused renal damage in rats, and the nephrotoxicity model was established successfully. There was no significant difference in serum CrE and BUN levels between HBPE (600 mg/kg) group and control group. The levels of serum BUN and CrE in CDDP + HBPE (150 mg/kg) group were slightly lower than those in CDDP group, but there was no statistical difference. The levels of BUN and CrE in CDDP + HBPE (300 mg/kg or 600 mg/kg) groups were significantly lower than those in CDDP group (p < 0.01).

Effects of HBPE and CDDP on serum BUN and CrE contents in rats. (A, BUN; B, CrE) **p < 0.01 vs. the control group, ##p < 0.01 vs. the CDDP group (n = 10).
Biochemical markers of oxidative stress The results of MDA, an indicator of lipid peroxidation level, are shown in Fig. 3. The content of MDA in kidney tissue of CDDP group was significantly higher than that of control group (p < 0.01). There was no significant change in the content of MDA between the HBPE group and the control group. The content of MDA in renal tissue of CDDP + HBPE (150 mg/kg) group was slightly higher than that of CDDP group, but there was no statistical significance. Compared with CDDP group, the level of MDA in renal tissue of CDDP + HBPE groups (300 mg/kg or 600 mg/kg) decreased significantly (p < 0.05 or p < 0.01).

Effect of HBPE on MDA content in CDDP induced rat kidney. **p < 0.01 vs. the control group, #p < 0.05, ##p < 0.01 vs. the CDDP group (n = 10).
As shown in Fig. 4, the level of GSH in renal tissue of CDDP group was significantly lower than that of control group (p < 0.01). There was no significant change in the level of GSH between the HBPE group and the control group. The level of GSH in renal tissue of CDDP + HBPE (150 mg/kg or 300 mg/kg) group was slightly higher than that of CDDP group, but there was no statistical significance. Compared with CDDP group, the level of GSH in renal tissue of CDDP + HBPE (600 mg/kg) group was significantly higher (p < 0.01).

Effect of HBPE on GSH content in CDDP induced rat kidney. **p < 0.01 vs. the control group, ##p < 0.01 vs. the CDDP group (n = 10).
As shown in Fig. 5, the activity of SOD in renal tissue of CDDP group was significantly lower than that of control group (p < 0.01). There was no significant change in SOD activity between HBPE group and control group. The activity of SOD in renal tissue of CDDP + HBPE (150 mg/kg) group was slightly higher than that of CDDP group, but there was no statistical significance. The activity of SOD in CDDP + HBPE (300 mg/kg and 600 mg/kg) group was significantly higher than that in CDDP group (p < 0.01).

Effect of HBPE on SOD activity in kidney induced by CDDP in rats. **p < 0.01 vs. the control group, ##p < 0.01 vs. the CDDP group (n = 10).
Glycometabolic enzymes in mitochondria MDH activity is shown in Fig. 6. CDDP could significantly decrease the activity of MDH in renal tissue (p < 0.01). There was no significant change in the level of MDH activity between the HBPE group and the control group. The activity of MDH in kidney tissue of CDDP + HBPE (150 mg/kg) group was slightly higher than that of CDDP group, but there was no statistical significance. Compared with CDDP group, the activity of MDH in CDDP+HBPE (300 mg/kg or 600 mg/kg) group was significantly increased (p < 0.05 or p < 0.01).

Effect of HBPE on MDH activity in CDDP induced rat kidney. **p < 0.01 vs. the control group, #p < 0.05, ##p < 0.01 vs. the CDDP group (n = 10).
LDH activity is shown in Fig. 7. CDDP significantly increased the activity of LDH in renal tissue (P < 0.01). Compared with the control group, there was no significant change in the level of LDH activity in HBPE group. The activity of LDH in renal tissue of CDDP + HBPE (150 mg/kg or 300 mg/kg) group was slightly lower than that of CDDP group, but there was no statistical difference. Compared with CDDP group, LDH activity in CDDP+ HBPE (600 mg/kg) group decreased significantly (p < 0.01).

Effect of HBPE on LDH activity in CDDP induced rat kidney. **p < 0.01 vs. the control group, ##p < 0.01 vs. the CDDP group (n = 10).
As shown in Fig. 8, CDDP alone showed a significant increase in HK activity in renal tissue (p < 0.01). Compared with the control group, there was no significant change in the level of HK activity in HBPE group. Compared with CDDP group, the level of HK activity in CDDP+HBPE (300 mg/kg, 600 mg/kg or 1200 mg/kg) group decreased significantly (p < 0.01).

Effect of HBPE on HK activity in CDDP induced rat kidney. **p < 0.01 vs. the control group, ##p < 0.01 vs. the CDDP group (n = 10).
Histopathological results As shown in Fig. 9, H&E staining section of renal histology showed that the renal tissue structure of rats in the control group and HBPE group was clear, and the glomerular and renal tubules were normal. In the CDDP group, glomerulus atrophy, renal tubular epithelial cell degeneration, renal interstitial lymphocyte infiltration and transparent tube type could be seen in the renal tubular lumen. In the CDDP+HBPE (150 mg/kg or 300 mg/kg) group, individual glomerular atrophy was observed, and transparent tubules were found in the lumen of renal tubules, which was improved compared with the CDDP group. In the CDDP+HBPE (600 mg/kg) group, the renal tissue damage was significantly reduced, and there were no abnormalities except mild glomerular hyperemia and turbidities in some epithelial cells of proximal convoluted tubules. This shows that HBPE can effectively reduce CDDP-induced renal pathological injury in rats.

Effect of HBPE on kidney histopathology in CDDP induced rat nephrotoxicity. (A) Control group (H&E, 200×). (B) HBPE (600 mg/kg) group (H&E, 200×). (C) CDDP group (H&E, 200×). (D) HBPE (150 mg/kg) +CDDP group (H&E, 200×). (E) HBPE (300 mg/kg) + CDDP group (H&E, 200×). (F) HBPE (600 mg/kg) +CDDP group (H&E, 200×).
As a tumor chemotherapy drug, CDDP is excreted mainly through the kidney, so its renal toxicity is the most obvious. It can cause kidney damage and acute renal failure caused by reactive oxygen species, tubulointerstitial inflammation and apoptosis induction (Hu et al., 2021; Lu et al., 2020). The main cause of renal toxicity induced by CDDP in rats was renal oxidative damage caused by oxidative stress. CDDP can not only produce large amounts of oxygen free radicals in the renal cortex, but also inhibit the activity of antioxidant enzymes or reduce the free radical scavenger in the tissue, thus damaging membrane protein and mitochondrial function, resulting in renal dysfunction and pathological structural changes (Lu et al., 2020; Wang et al., 2018). The concentrations of serum BUN and CrE depend on the catabolism of nitrogen in the body and the excretion capacity of the kidney. The concentrations of BUN and CrE can reflect the damage degree of glomerular filtration rate function to a certain extent, and the change of organ coefficient can better reflect the comprehensive toxic effect of toxins on the organ. Therefore, kidney index, serum BUN and CrE were used as markers of renal injury in rats in this study. The results of this study showed that intraperitoneal injection of CDDP (7.5 mg/kg) in healthy adult male rats significantly increased kidney index, serum BUN and CrE content, indicating that CDDP caused obvious kidney injury in rats.
SOD is an important antioxidant enzyme in the body, which plays an important role in scavenging free radicals and protecting the body from oxidative damage. It plays an important role in scavenging free radicals and protecting the body from oxidative damage (Valentovic et al., 2014; Zhang et al., 2020). GSH is a small molecule peptide containing sulfhydryl group, which has important antioxidant and integrative detoxification effects and is an important free radical scavenger in the body (Chen et al., 2019; Dong et al., 2020). MDA is the final decomposition product of lipid peroxides in the body. When the metabolism of free radicals is disturbed, excess oxygen free radicals will result in damage to renal tubular epithelial cells, and increased reactive oxygen species will lead to lipid peroxidation and degradation into MDA (Dillioglugil et al., 2010; Köroğlu et al., 2019). MDA is a characteristic index to judge the degree of lipid peroxidation in the body (Tsikas, 2017). The results of this study showed that after intraperitoneal injection of CDDP (7.5 mg/kg), MDA content in the renal cortex of rats were increased, and GSH content and SOD activity were significantly decreased, confirming that CDDP caused renal oxidative damage, suggesting that renal toxicity induced by CDDP was related to oxidative stress. The results are consistent with previous findings by researchers.
Mitochondria are the main sites of ATP synthesis, electron transport and oxidative phosphorylation (Mei et al., 2020). The normal physiological function of kidney, including the reabsorption of renal tubules, requires the effective energy provided by various metabolic pathways, among which the generation of ATP by mitochondrial aerobic respiration is the main way of energy generation in the body (Tait and Green, 2012). CDDP can directly attack mitochondrial DNA, or induce the generation of a large number of free radicals to attack mitochondria, resulting in changes in the activity of ion transporters, thereby disrupting the ionic balance of cells, changing mitochondrial permeability, reducing transmembrane potential, and thus affecting the mitochondrial function of renal tubular epithelial cells, resulting in ATP production disorders (Guan et al., 2021; Li et al., 2020). In this study, the indexes of carbohydrate metabolism enzymes (MDH, LDH, and HK) were studied to evaluate the energy metabolism function of mitochondria. MDH widely exists in mitochondria as a marker of the tricarboxylic acid cycle. LDH and HK exist in cytoplasm and are marker enzymes in glycolysis. The results of this study showed that CDDP could significantly increase the activity of LDH and HK, while significantly decrease the activity of MDH in the renal cortex of rats. These results indicated that CDDP reduced the activity of metabolic enzymes in the tricarboxylic acid cycle pathway in mitochondria, which mainly provided energy, and the energy needed by cells needed to be provided through other channels. Therefore, the activity of glycolysis metabolic enzymes increased, indicating that CDDP induced significant mitochondrial damage in rats.
Polyphenols are a kind of active substances with various physiological functions widely existing in the plant kingdom. They have strong ability of scavenging free radicals and can also play an anti-oxidation role by inhibiting oxidase and complexing transition metal ions (Ge et al., 2021). Highland barley contains a lot of polyphenols and has strong antioxidant activity (Shen et al., 2016). Yang et al. demonstrated that malt extract prevented the decrease of antioxidant enzyme activities, decreased liver and brain MDA levels and carbonyl content, and improved total antioxidant capability in d-galactose-treated mice (Qingming et al., 2009). Quan et al. showed that pretreatment with free phenolic extract from raw barley was effective in the prevention of oxidative stress and liver damage induced by CCl4 in rats, as revealed by the marked decrease in the hepatic lipid peroxidation content, the reduced serum AST, ALT, TC, TG and AKP levels, the enhanced hepatic SOD, CAT and GPx activities (Quan et al., 2018). Shen et al (2016). have demonstrated that the antioxidant defense system and antioxidant gene expression were significantly improved in mice gavaged with polyphenols extracted from black highland barley compared to mice on a high-fat diet (Shen et al., 2016). This study was conducted to investigate the effect of HBPE at 150 mg/kg, 300 mg/kg and 600 mg/kg doses 15 d in advance on renal toxicity induced by CDDP in rats. The results showed that pretreatment with higher dose of HBPE could significantly reduce the levels of BUN and CrE in serum induced by CDDP, inhibit the depletion of GSH induced by CDDP in renal cortex, increase the content of MDA and decrease the activity of SOD. It is suggested that HBPE can alleviate the renal toxicity induced by CDDP. The mechanism may be to reduce oxidative stress response by reducing free radicals, increasing antioxidant enzyme activity and antioxidant content, thereby inhibiting the formation of lipid peroxides and alleviating oxidative damage. In addition, HBPE pretreatment can also slow down the decrease of MDH activity and the increase of LDH and HK activity induced by CDDP, the mechanism may be that HBPE alleviates mitochondrial damage by inhibiting oxidative stress in the body. These results suggest that HBPE can effectively reduce renal toxicity induced by CDDP, which is closely related to its anti-oxidation and anti-mitochondrial damage. With the increase of HBPE concentration, it has a more significant protective effect on CDDP induced renal injury, indicating that HBPE has a dose effect, and high-dose HBPE pretreatment can effectively reduce CDDP induced renal toxicity. In addition, There is possibility that one of polyphenols shown in Table 1 reduces CDDP-induced oxidative damage, or that these multiple components act synergistically. Elucidation of these possibilities needs to be examined in the future.
This study showed that HBPE had a protective effect on renal toxicity induced by CDDP. This protective effect of HBPE is related to its anti-oxidation and anti-mitochondrial damage. Therefore, HBPE can be used in combination with CDDP in cancer patients to improve kidney injury caused by CDDP.
Acknowledgements This work was supported by Beijing Natural Science Foundation (NO.7163211).
Conflict of interest The authors declared that they have no conflicts of interest to this work.
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.