| Edited by Hirokazu Inoue. Yoshito Sadaie: Corresponding author. E-mail: ysadaie@molbiol.saitama-u.ac.jp |
Bacillus subtilis is refractory to infection by phage SP10, which can multiply in Bacillus licheniformis. Mutational inactivation both of nonA and nonB (non-permissiveness) genes render B. subtilis cell permissive to infection by SP10 (Saito et al., 1977). The nonA is located close to rpoB by PBS1-mediated transduction, whereas the location of nonB is not separable by transformational cross from the intrinsic restriction gene BsuMR in the purB region (Saito et al., 1977). Although nonB is believed to be an allele of BsuMR, the precise mutational structure of nonB has not been elucidated so far.
The intrinsic restriction and modification system BsuM of B.subtilis Marburg consists of two sets of genes BsuMM and BsuMR located in the prophage 3 region (Kasahara et al., 1997; Kunst et al., 1997). BsuMM consists of ydiO and ydiP, and BsuMR is composed of ydiR, ydiS and ydjA (Ohshima et al., 2002). Therefore nonB must be a mutation(s) in ydiR, ydiS or ydjA. The target DNA sequence of BsuM is considered to be the same as that of XhoI, CTC*GAG (Bron et al.,1988; Jentsch, 1983), in which the 3’ cytosine (indicated by asterisk) is the acceptor for BsuM modification (Jentsch, 1983). Multiple presence of the unmethylated target sequences are required for effective restriction by BsuMR (Bron et al., 1988). Therefore the SP10 phage genome is assumed to contain a multiple number of XhoI sites.
In this communication, we have determined the mutational sites of nonA and nonB. All the nonA strains were cured of the intrinsic temperate phage SPβ (Warner et al., 1977) of B.subtilis Marburg, which possibly interferes with infection by SP10 phage. The nonB strain on the other hand contained one nonsense mutation in ydiR, which is a component gene of the BsuMR (Ohshima et al., 2002).
Bacterial strains and plasmids used are described in Table 1. B.subtilis phage used was SP10 provided by Y.Itoh (Inatsu et al., 2002), which was from H.W.Ackerman (Ackerman et al., 1995).
 View Details | Table1. Bacterial strains and plasmids used |
Conventional DNA mediated transformation was performed as described elsewhere (Ohshima et al., 2002). NonA+ transformants were selected as colonies appearing on Luria-Bertani (LB) broth agar, on which SP10 phages (ca. 109), grown in RM125, were spread before plating the transformed culture. Transformation with lysed protoplasts as donor DNA developed by Akamatsu and Sekiguchi was performed as described elsewhere (Akamatsu and Sekiguchi, 1987). After serial dilution of the protoplast in SMM buffer, one-tenth vol-ume of the protoplast sample was added to one volume of competent culture. Donor strains for conventional transformation or transformation with DNA from lysed pro-toplasts carry the integrative plasmid pMUTIN2 (em) (Vagner et al., 1998). Disruptants are described in the Table 2 and in the web site of BSORF (http://bacillus. genome.ad.jp/).
 View Details | Table 2. Disrupted genes carried by the strains, from which protoplasts and their DNA were prepared for transformation. |
The number of SP10 phages was counted as the number of plaques in a soft agar (0.8%) containing cells of the indicator strain and SP10 phages. The soft agar was overlaid on nutrient agar (1.5%) and incubated at 37°C. Both agar contained LB broth. SP10 DNA was extracted as follows. The culture of RM125(NonA– NonB–) or RIK137(ΔSPβ ydiS::pMUTIN2) in LB broth was infected by SP10 phages and cell debris was discarded by centrifugation. After 10% PEG(6000) and 0.5M NaCl precipitation, phages were suspended in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) containing 5 mM MgSO4. The sample was treated with DNase and RNase. EDTA (50 mM) and SDS (1%) were added to the sample to lyse phage. The sample was treated with phenol. DNA solution was made 0.3 M with sodium acetate, and DNA was extracted with glass rod after ethanol addition.
Disruptions of the prophage SPβ genes, mtbP, ligB, yonB, yopA and yomD, the skin element genes yqxG and yqaP, and the PBSX gene xkdK were carried out as described elsewhere (Ohshima et al., 2002) with plasmid pMUTIN (Vagner et al., 1998) and with DNA fragments synthesized by PCR using primer pairs 5’aaga-agcttagtggaattggTGCAT/5’GGAGGATCCTCATA-AGTAATTAAAT for mtbP, 5’AAGAAGCTTACGACGAGA-ACTATATCACTG/5’GGAGGATCC TAGCTGCAATTGATTGACCGT for ligB, 5’AAGAAGCTTACTGTGAAAATCAA-AGGCTTG/5’GGAGGATCCTAAATCAACACCAGAGCCTGT for yonB, 5’AGCAAGCTTTTCGAATTTGAA/5’GGAGGATCCAATAGTCATATCGCTTATCCA for yopA, 5’AAGAAGCTTTAGCTCAGAATAACTACATTC/5’GGAGGAT-CCTCACCTTTAAAGAAGTTGCTG for yomD, 5’AAGAAG-CTTTCAACAGATGCAACTGGTAAG/5’GGAGGATCCAC-TCAGTCTCGCCAAGCGTAT for yqxG, 5’AAGAAGCTTA-TTGGAGTTAGTGTTTCGCAC/5’GGAGGATCCTACGCT-AATTACGTTCTCATT for yqaP, and 5’AAGAAGCTTCGTGCAGGCATTTATTTTAAC/5’GGAGGATCCCGCCATAT-ACTGCAGTTGCTT for xkdK.
Overexpression of ydiR gene was induced by adding IPTG (1 mM) to the culture of the strain carrying, at aprE locus, pAPNC213 plasmid (Morimoto et al., 2002) with ydiR gene fused to a Pspac promoter. Pspac-ydiR fusion gene on the plasmid pAPNC213 was constructed as follows. Primer pair for PCR synthesis of ydiR gene was 5’gTCgTCgACAGATTTGAGGTTGATATCTTT/5’GGAGGATCCTTAGTTATTTTCAAAG-TCTTC. PCR product was digested with SalI and Baminto pAPNC213, which carries split tains moter. pAPNC213-and was used to transform UOT1012 and then PS9 to spectinomysin resistance.
A DNA segment lacking SPβ was made by a double-cross over recombination using a linearized recombinant plasmid with two DNA fragments flanking the attachment site of SPβ. These DNA fragments were am-plified by PCR with UOT1285 genome DNA and two primer pairs 5’-CGGTCTGAGAATTCAACTTAAATTCACGC/5’-ACACAGCTGTGTACTGATATTAATGACA-TGC, and 5’-TTTCAGCTGAAAGCTGTATCTCCTGTAACAC/5’TACGTCGACGTATGTAGACATTGTGTCAGGC containing EcoRI, PvuII or SalI restriction site. Resulting PCR products were digested with these restriction en-zymes, and cloned into the EcoRI-SalI region of pBR322M (a pBR322- derivative deleted of a DNA fragment by digestion with PvuII and Bst1107I) to obtain pSB322M. Then pSB322M was digested with PvuII and ligated with a Cmr gene cassette derived from pC194 (Horinouchi et al., 1982) through pCBB31(Nanamiya et al., 2004; Yamada, 1989). The recombinant plasmid pSB322MCm was linearized with ScaI and used to transform UOT1285 (SPβ+). A Cmr transformant was selected and named RIK135 (ΔSPβ::cm). Chromosomal DNA of RIK135 was used to transform Leu– RIK1010 to construct Cmr Leu– RIK136 (ΔSPβ::cm). To remove the cm gene from RIK136, two DNA fragments (PCR synthesized Leu+ DNA and ScaI-digested pSB322M linear DNA) were used to transform RIK136, and a Leu+ Cms transformant was selected by congression and named RIK137 (ΔSPβ). The Leu+ DNA fragment (about 6.6 kb) was synthesized by PCR with a primer pair 5’-GCTGGAAAAACAGTAGCGGTTATCGGGTAC/5’-CTTGCGTCGACCAGCAATTTCGGCTGGAACG and UOT1285 genome DNA. The deletion of SPβ was confirmed by PCR and by sensitivity for SPβC29, a clear mutant of SPβ (isolated by F.Kawamura). 168ΔSPβcm (trpC2 ΔSPβ cm) was made by transformation of 168 to Cmr by DNA from RIK135 (ΔSPβ cm).
Confirmation of the presence or the absence of SPβ by PCR was performed with primers, 1(yodUF): AT-GCCTAAACAGCAAACAGCA, 2(SPβ yotLR):TGTCTGCGCTCTTTAAAGTTC, 3(SPβ yokAF):ACGAATCTAA-ACGCATACGAA, 4(ypqPR):TGCCTTTTTCTCTTGATGCAA. Their configurations are shown in Fig. 2. yodU and ypqP are located outside of attachment site of SPβ. yotL and yokA are located at the right and left side ends of SPβ (Kunst et al., 1997).
To find mutated bases of the nonB gene, we have sequenced five overlapping DNA fragments of about 1 kb covering the prophage 3 region of nonA nonB strain PS9. DNAs were PCR synthesized by using template chromosomal DNA of PS9 with primer pairs 5’GGCTCGCAGTCACAACAA/5’CCCTTTCTTCCC-CGTTAC, 5’CCTTCTGTGGACGATTCG/5’CTCTTCCTC-ACCGCTTAA, 5’GGGAAGTGGGAAAGATCT/5’ACCACCGAGTACACGTAC, 5’GATGGCTCGGATAACATT/5’AC-TTCCTCCCATACTCTG, 5’GGCATTGAAAACCGCAAT/ 5’GAGAGAGAAGCGGTGGAT. Each primer pair contained a SP6 and T7 promoter tag sequence pair 5’atttaggtgacactatagaatac/5’taatacgact cactataggg at its 5’ ends.
As nonA is reported to be located in the region of rpoB(rif) (Saito et al., 1977), conventional transformation of Non– (nonA nonB) strain to Rifr Non+ (nonA+ nonB) with biochemically purified DNA from strain UOT1871 carrying rif1728(rpoB)(Murayama et al., 2004) was performed. Non+ transformants were selected as SP10 phage (grown in RM125) resistant colonies as described in the Materials and Methods section. However no Rifr Non+ transformants were obtained. Next, similar experiments were performed with DNA from strains carrying integrative plasmid pMUTIN2 (em) in the 21 orfs located within 20 kb around the rpoB gene. However no Emr Non+ transformants were obtained. Therefore with both transformations we could not find linkage of the nonA mutation to rif (rpoB).
Chromosomal DNA from gently lysed protoplast cell carries more genetic markers than biochemically purified DNA (Akamatsu and Sekiguchi, 1987). We performed transformation from Ems to Emr of PS9 strain (nonA nonB) with chromosomal DNA prepared from protoplasts of 220 disruptant strains carrying integrative plasmid pMUTIN2 (cm) (Table 2) described in BSORF (http://bacillus.genome.ad.jp/). They are NonA+ NonB+ strains. Disrupted genes are located at every 20kb along the whole chromosome (Kunst ). As shown in Fig. 1, 13 donor strains carrying integrative plasmid pMUTIN2(em) in the genes, ydeO, ydfI, ydfJ, ydfK, ydgC, ydiL, ydjB, yeaC, yonB, yomD, ywkE, ywcH, and yxkA showed higher transformation frequency (co-transfer index more than 20) of Non– (nonA nonB) to Emr Non+(nonA+ nonB or nonA nonB+). Several Emr Non+ colonies from each transformation were taken and examined for their phage resistance. They were used as indicator strains for plaque forming ability of SP10 phage. Transformants obtained in transformation with ydeO, ydfI, ydfJ, ydfK, ydgC, ywkE, ywcH, and yxkA disruptant DNA were SP10 phage sensitive. On the other hand, transformants with DNAs from donor strains carrying pMUTIN2 (em) in the genes in the regions of prophages 3 (ydiL, ydjB and yeaC) and SPβ (yonB and yomD) showed SP10 phage resistance. These results suggested that nonA and nonB mutations are located in these regions. As described below, since nonB is located in the prophage 3 region, nonA must be located in the SPβ region.
 View Details | Fig. 1. Transformation, with lysed protoplasts as donor DNA, of Non–(nonA nonB) strain to Non+(nonA+ nonB or nonA nonB+). Donor strain (isogenic strain of 168 Marburg carrying trpC2) possesses disrupted gene with the integrative plasmid pMUTIN2 (em) described in the Table 2. Disrupted genes are placed on abscissa in clockwise order from chromosome replication origin. Co-transfer index on ordinate indicates the ratio, 100 x Emr Non+/ (Emr Non– + Emr Non+). Recipient strain was PS9 (nonA nonB). The locations of rpoB, BsuM and SPβ are shown. O and T indicate the origin and the terminus of chromosome replication respectively. |
It is possible that SPβ interferes with multiplication of SP10, simply because of the immunity established by lysogenic SPβ against infecting SP10. The presence or absence of SPβ (Kunst et al., 1997; Warner et al., 1977) in Emr Non+ transformants was confirmed by PCR (Fig. 2). PCR synthesis of DNA fragments covering the outside genes of SPβ and the right or left end genes on SPβ (PCR with primer pairs 1 and 2 in Fig. 2c or 3 and 4 in Fig. 2d), or PCR synthesis of DNA fragment covering outside genes of SPβ (PCR with a primer pairs 1 and 4 in Fig. 2b) were performed by using template DNA from the wild type strain, nonA+ transformant, or nonA strains as well as from strains cured of SPβ. The presence of SPβ is shown in Fig. 2C as a DNA band of 1.5 kb long, and in Fig. 2d as a DNA band of 1.3 kb long, and is not shown in Fig. 2b, as SPβ (ca. 134kb) (Lazarevic et al., 1999) is too long to be synthesized by PCR. When the chloramphenicol resistance cassette of pSB322Mcm plasmid was replaced for SPβ, a DNA band of 2.2 kb long was shown in Fig. 2b. A PCR product of 1 kb long is shown in Fig. 2b, when SPβ phage was cured. PCR products indicating the presence of SPβ were observed only from nonA+ strains (Fig. 2, lanes 1, 4, 6, 9) but not from nonA strains and strains cured of SPβ (Fig. 2, lanes 2, 3, 5, 7, 8, 10, 11, 12, 13). The Trp+ NonA– transformant of ydiS disrupted strain BSU4 (ydiS::em trpC2) with protoplast DNA of nonA strain YS11 did not show the above PCR product (Fig. 2, lane 8). These results indicate that the wild type strain carries SPβ and the nonA mutant strains or nonA transformants do not carry SPβ. Furthermore, all the SPβ-free strains carrying the nonB mutation were permissive for the infection and growth of SP10 phage (Fig. 2e).
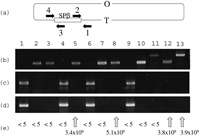 View Details | Fig. 2. PCR product of SPβ and permissiveness for SP10. (a) Location of SPβ on B.subtilis chromosome and configuration of primers used. (b) Agarose electrophoresis of PCR products with primer pairs 1 and 4, and template DNA from wild type strain 168(lane 1), nonA strains HLL3g (lane 2) and YS11(lane3), nonB strain UOT1012(lane 4), nonA nonB strain PS9(lane 5), emr nonA+ nonB– transformat of PS9 with DNA from yonB::pMUTIN2(em) (lane 6), emr nonA– nonB+ transformant of PS9 with DNA from ydiL::pMUTIN2(em)(lane 7), NonA– Trp+ transformant of ydiS::em strain BSU4 with DNA from YS11(lane 8), NonA+ Trp+ transformant of ydiS::em strain BSU4 with DNA from YS11 (lane 9), ΔSPβ strain RIK137 (lane 10), ΔSPβ cm strain 168(ΔSPβ cm) (lane 11), Trp+ ΔSPβ transformant of ydiS::em strain BSU4 with DNA from RIK137(lane 12), ΔSPβ cm transformant of ydiS::em strain BSU4 with DNA from 168 ΔSPβ cm (lane 13). (c) Agarose electrophoresis of PCR products with primer pairs 1 and 2, and template DNA from strains described above. (d) Agarose electrophoresis of PCR products with primer pairs 3 and 4, and template DNA from strains described above. (e) Plaque forming unit per ml of SP10 phage using indicator strains from which template DNAs were extracted for PCR described above. |
We confirmed the causality of ΔSPβ and defective BsuMR for a NonA– NonB– phenotype by using strains cured of SPβ. By introducing ydiR(em), ydiS(em), or ydjA(em) disrupted genes (Ohshima et al., 2002) into ΔSPβ strain RIK137, all the transformant strains became permissive to SP10 infection, while similar transformants of SPβ+ UOT1285 were not permissive (Table 3). Permissiveness for SP10 phage therefore requires curing of SPβ and the defective function of at least one of the ydiR, ydiS or ydjA genes, whose products constitute BsuMR. Furthermore, chlora-mphenicol resistant (cm) transformants of ydiR, ydiS or ydjA strain with DNA from ΔSPβ cm were permissive for SP10 infection (data not shown).
 View Details | Table 3. Combination of ΔSPβ and ydiR, ydiS or ydjA and permissiveness for SP10 growth |
The frequency of transformation, with DNA from lysed protoplasts, of wild type (SPβ+) strain to nonA (ΔSPβ) was higher than the frequency of similar transformation of nonA (ΔSPβ) to wild type (SPβ+). This is probably due to excision of single strand DNA bearing large (134kb)(Lazarevic et al., 1999) SPβ in hetero-duplex intermediate DNA formed during transformation.
Since nonB is responsible for restriction of phage infection (Saito et al., 1977; Witmer et al., 1981), we directly sequenced five overlapping DNA fragments of about 1 kb covering the entire BsuMR region of the nonA nonB strain PS9. We found one nonsense mutation TAA and one synonymous mutation TCA in ydiR gene (69th codon CAA and 228th codon TCG respectively) of the nonA nonB strain PS9 (Fig. 3). There was no mutation found in the ydiS and ydjA of the above strain.
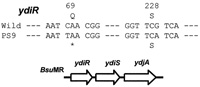 View Details | Fig. 3. DNA sequence of ydiR gene of the B.subtilis nonA nonB strain. DNA sequence of the wild type strain is described above that of the nonA nonB strain PS9. The number indicates the number of the codons where mutations were found. The nonB mutation possesses one nonsense mutation in the 69th codon (CAA to TAA) of ydiR and one synonymus mutation in the 228th codon (TCG to TCA) of ydiR gene. |
We next confirmed whether or not the wild type ydiR gene complemented the above nonB mutation. Single copy ydiR gene was cloned in pAPNC213 (Morimoto et al., 2002) under Pspac promoter and introduced at aprE locus of nonA nonB strain PS9 and induced by IPTG. As shown in Table 4, with 1mM IPTG, PS9 strain carrying pAPNC213-ydiR showed NonB+ phenotype, i.e., non-permissiveness to SP10 infection. Therefore, these results indicated that above nonsense mutation in ydiR is responsible for the nonB phenotype.
 View Details | Table 4. Complementation of nonB by ydiR |
DNA extracted from SP10 phage grown in the BsuM deficient strain RM125(Ohshima et al., 2002), which was also cured of SPβ (data not shown), was digested with XhoI and we found more than 20 fragments. On the other hand, SP10 DNA extracted from phage lysate derived from a BsuMM+ strain, which carried disrupted ydiS and was cured of SPβ, was more refractory to digestion by XhoI (data not shown). This is the first indication that the SP10 genome contains multiple XhoI sites, which are target sites of the BsuM system. Therefore SP10 phage is not able to multiply in B.subtilis cell with an intact BsuM system.
An explanation for probable interference of prophage SPβ with SP10 multiplication is that wild type SPβ repressor may affect expression of the SP10 phage genes leading to non-permissiveness of phage growth. Thermosensitive induction of SPβ due to temperature-sensitive mutants of SPβc2 (Rosental et al., 1979) suggested involvement of the repressor YomJ (Lazarevic et al., 1999) in prophage maintenance. Another explanation is that some gene(s) on the prophage SPβ is expressed and may interfere with the some process(s) of infection, expression, replication and packaging of SP10 genome. Examples of the expressing genes are the sspC gene encoding a small acid-soluble protein integrated in spore, and the genes for the SPβ bacteriocin YolG (betacin) and bacteriocin-exporter YokH (Lazarevic et al., 1999).
On the other hand, SPβ does not interfere transformation of B.subtilis Marburg strain with plasmid pHV1401 harboring multiple BsuM target sites. Plasmid-mediated transformation requires only a defect (nonB) in BsuMR (Bron et al., 1988; Oshima et al., 2002).
We are grateful to R.H.Doi for critically reading the manuscript and to Y.Itoh for providing SP10 phage. This work was partially supported by a Grant-in-Aid for Scientific Research on Priority Area from the Ministry of Education, Science, Sports and Culture of Japan.
|