| Edited by Kiyotaka Okada. Masayoshi Shigyo: Corresponding author. E-mail: shigyo@yamaguchi-u.ac.jp |
Bunching onion (Allium fistulosum) has been cultivated in several countries of eastern Asia from ancient times (Inden and Asahira, 1990). In A. fistulosum, water-soluble carbohydrates account for approximately 66% of the dry weight in the leaf. These carbohydrates include glucose, fructose, sucrose and fructans (Fenwich and Hanley, 1990; Darbyshire and Steer, 1990). The amount of each sugar in the leaf blade greatly affects the quality of bunching onion in terms of its texture, taste and flavor.
We previously developed eight Allium fistulosum - shallot (A. cepa) monosomic addition lines that display morphological and physiological characteristics different from the A. fistulosum parent (Shigyo et al., 1997a), likely due to alien gene or genes on the extra chromosome from shallot. An investigation of the carbohydrates in the Allium monosomic additions should reveal changes in the sugar content of A. fistulosum, as well as to show the chromosomal locations of the shallot genes important for carbohydrate metabolism. In the present study, the sugar contents in a complete set of A. fistulosum – shallot monosomic additions were evaluated monthly to determine the effect of a single alien chromosome from shallot on carbohydrate production in leaf-bunching onion.
The plant materials were a complete set of Allium fistulosum – A. cepa monosomic additions (2n = 2x + 1 = 17, FF+1A-FF+8A) and a control plant, Japanese bunching onion (A. fistulosum L. cv. Kujyo-Hoso, 2n = 2x = 16, FF). The monosomic additions were grown in an experimental field at Yamaguchi University (34°N, 131°E), over two-years (Jan. 2002 – Dec. 2003). Cultivation and fertilizer applications were carried out according to the procedures of Shigyo et al. (1997a).
Extraction of the sugar from leaf blade tissues and quantitative analysis were done according to the methods of Yamauchi et al. (2000) with minor modifications. Sugars from fresh leaf blades (2.0 g) were extracted with hot 73% ethanol (final concentration 70% EtOH) for 15 min. The 70% EtOH extracts were analyzed by the Somogyi-Nelson method to determine the reducing and non-reducing sugar contents and by the HPLC method to determine the glucose (Glc), fructose (Fru) and sucrose contents.
Data on the percentage of non-reducing sugar (NRS) contents in total sugar by month for 24 months in the eight addition lines and Allium fistulosum were used for one-way analysis of variances (ANOVA). Dunnett's test was employed to compare the NRS content rate between A. fistulsoum and each monosomic addition. Regression analysis was carried out to clarify the relationship between the reducing sugar (RS) and monosaccharide (Glc and Fru) content rates as well as between the NRS and sucrose content rates. Another regression analysis was conducted to find the relationship between the data obtained in 2002 and those obtained in 2003. Statistic analyses of the data were performed using SPSS 11. 5 software with advanced models (SPSS, Japan).
As shown in Fig. 1, all the plant materials tended to accumulate RS into the leaf blade in the summer and autumn seasons. Except for FF+2A, every type of monosomic addition had a tendency to accumulate NRS in the winter leaf blades. FF+2A produced very little non-reducing sugar throughout the two-year study. FF+8A showed that the alien 8A chromosome caused the value of the NRS to spur in winter.
 View Details | Fig. 1. The year-round variations of reducing and non-reducing sugar contents in a complete set of monosomic additions (FF+1A-FF+8A) compared with A. fistulosum (FF). The mean value in each month from Jan. 2002 to Dec. 2003 was shown in this figure. |
Biennial numeric data on the percentage of NRS in total contents are shown in Table 1. One-way ANOVA revealed significant differences (at the 1% level) in the monthly percentages of NRS as well as differences among plant materials (Table 2). Dunnett’s test showed non-significant differences between each of the monosomic additions, except between the FF+2A and the control (Table 3). The results of regression analysis using data over two years (as shown in Table 4) revealed a correlation between 2002 and 2003 in FF+2A and FF+8A. Based on these results, shallot chromosome 2A carries genes which stimulate the decomposition of NRS or genes that inhibit NRS synthesis. Genes that stimulate the synthesis of NRS are located on 8A.
 View Details | Table 1. Percentages of non-reducing sugar contents in total sugar by month in monosomic additions (1A–8A) and A. fistulosum (FF) |
 View Details | Table 2. Analysis of variance for percentages of non-reducing sugar contents in monosomic additions and A. fistulosum |
 View Details | Table 3. Dunnett multiple-comparison test for comparison of non-reducing sugar contents between A. fistulosum and each monosomic addition |
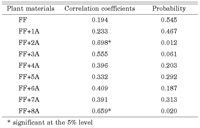 View Details | Table 4. Regression analyses between percentages of non-reducing sugar content for 2002 and 2003 in monosomic additions (FF+1A–FF+8A) and A. fistulsoum (FF) |
All plants constantly produced every month in 2002 one oligosaccharide (sucrose) as well as two monosaccharides (Glc and Fru). The sugar content for these three sugars showed little variation throughout 2002 (Fig. 2). Regression analyses comparing the two different data on sugar content obtained by the Somogi-Nelson and HPLC methods in A. fistulosum revealed a correlation between the RS and monosaccharide (Glc and Fru) content rates, but no correlation between the NRS and sucrose content rates (Fig. 3). Similar relationships between the RS and monosaccharide content rates were observed in monosomic additions FF+1A, FF+4A and FF+6A (Table 5). The NRS vs. sucrose content rates were not statistically significant, indicating the existence of other polysaccharides (e.g., scorodose) as NRS and other monosaccharides (e.g., galactose) as RS in leaf blades.
 View Details | Fig. 2. The year-round variations (in 2002) of fructose, glucose and sucrose contents in a complete set of monosomic additions (FF+1A-FF+8A) compared with A. fistulosum (FF). |
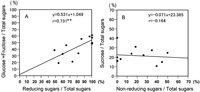 View Details | Fig. 3. Scatter diagrams showing the relationships between two different data sets on sugar contents obtained from the Somogyi-Nelson and HPLC methods in A. fistulosum. (A) High correlation between reducing sugar and monosaccharide (Glc and Fru) content rates. (B) No correlation between non-reducing sugar and sucrose content rates. ** significant at the 1% level |
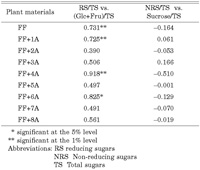 View Details | Table 5. Correlation coefficients between RS and monosaccharide content and between NRS and sucrose content in monosomic additions (FF+1A-FF+8A) and A. fistulsoum (FF) |
Mizuno and Kinpyo (1955) reported that the free sugars and storage carbohydrates in the leaf blade of bunching onion were Glc, Fru, sucrose, maltose and fructose-oligosaccharides. In the leaf blade, mucilages, which are well-hydrated gels of cellulose, hemicellulose, protopectin and water-soluble pectin, embed these free and storage sugars (Inden and Asahira, 1990). The eating quality of leaf-bunching onion improves under low temperatures because this mucilaginous complex increases. This complex is rich in oligosaccharide, pectin and other substances and recently has been recognized as a effective in treating digestion, wounds, festering sores and cholesterol, among other ailments (Inden and Asahira, 1990). In bunching onion, a limited number of studies have characterized fructose-oligosaccharides in the leaf sheath (Ernst et al., 1998). The result of a preliminary TLC analysis showed that monosaccharides to pentasaccharides were contained in the 70% ethanol extract from a leaf blade. The productivity of mucilages is related to NRS. FF+8A is overproducing line for NRS and produced higher mucilages, as compared to the depressed production line (FF+2A), which seldom produced mucilages. These plant materials are vital for elucidating the mechanism of the metabolic pathway for mucilages, including fructose-oligosaccharides.
Alien monosomic additions often showed desirable agronomic traits for improving the recipient species (Khush, 1973; Singh, 2003). Several effects of the extra chromosomes on morphology and fertility were previously reported for two complete sets of the Allium monosomic additions (Shigyo et al., 1997a, 1999). The alterations observed in the monosomic additions were qualitative and quantitative. Modifications were not always due to the interaction between genes of bunching onion and shallot, for instance, FF+5A produced the flavonoid kaempferol, the anthocyanin cyanidin-glucoside and their related compounds in the leaf sheath (Shigyo et al., 1997b). FF+1A was resistant to Puccinia allii, which causes the rust disease in A. fistulosum (Wako et al., unpublished data). This monosomic addition has been utilized to incorporate small segments of the shallot chromosome to A. fistulosum by radiation-induced translocations. Such a translocation procedure may be applicable for affecting NRS content in bunching onion because the differences found in this study were caused by the effects of homoeologous genes on the F and A genomes. Before applying this procedure, the number and localization of the genes related to this trait should be revealed on the chromosome 8A. Alternatively, we identified a disomic addition line carrying two 8A chromosomes in the selfed progeny of FF+8A (Shigyo et al., 2003). Further examinations of NRS and seed production ability for this disomic addition line will reveal the potential usefulness of this addition line as a breeding resource for a new variety of bunching onion.
In Allium cepa, the sucrose synthesized in the leaf blade is translocated to the leaf sheath and is the substrate for the accumulation of soluble carbohydrates in the bulb. Recently, the mode of inheritance for soluble carbohydrate production has been clarified by localizing candidate genes (McCallum et al., 2001) and QTLs (Havey et al., 2004) related to sucrose and soluble carbohydrate accumulations on an onion linkage map based on molecular markers (King et al., 1998). The combination of both a molecular marker map and a monosomic addition set will reveal the chromosomal locations of QTLs related to carbohydrate production in onion bulbs, as well as to an understanding of the localizations of the different QTLs on the same chromosome.
The authors thank Misses A. Kagawa, M. Nakashima and Y. Nakahara for their contributions to this study. The authors would like to thank Dr. M. J. Havey of USDA and the University of Wisconsin for critically reviewing this manuscript. The authors further express their gratitude to Mr. Lynn Hofstad of Hot Line for reviewing the manuscript regarding the English language.
|