| Edited by Hirokazu Inoue. Kazuo Yamamoto: Corresponding author. E-mail: yamamot@mail.tains.tohoku.ac.jp |
It has been argued that replication error rates vary depending on the replicating strand (Radman, 1998). Given that the synthesis of the two anti-parallel strands of duplex DNA occurs only in the 5’→3’ direction, each of the strands are thought to be copied by different processes and proteins (Kornberg and Baker, 1992). This asymmetry creates the potential for differences in error rates between the leading strand and lagging strand rep-lication machinery. For example, a preference for the lagging strand was observed with respect to the induction of frameshift mutations (Veaute and Fuchs, 1993; Iwaki et al. 1996) and deletion mutations (Trinh and Sinden, 1991), using plasmid-containing target genes. Fija-lkowska and colleagues developed strains containing a series of lacZ reversion alleles on the chromosome in two orientations with respect to the origin of replication (oriC) and observed directionality associated with spontaneous base substitutions (Fijalkowska et al., 1998) and frameshifts (Gawel et al., 2002a), but an absence of strand bias in UV-induced base substitutions (Gawel et al., 2002b).
During the past several years, we have developed a system to systematically study mutagenesis by employing the endogenous tonB gene of E. coli (28 min position of the E. coli linkage map) as a target (Kitamura et al., 1995; Uematsu et al., 1999; Mashimo et al., 2004; Ohnishi et al., 2004). In an extension of these studies, we inserted the tonB gene in the reverse orientation with respect to oriC. If the leading strand and lagging strand modes of replication differ in the incidence of replication fidelity, differences in base substitution, deletion and frameshift mutations should be observed between the two tonB orientations. In this study, we constructed an additional pair of polA1 strains. DNA polymerase I, encoded by the polA gene, consists of a single polypeptide with two major domains (Brutlag et al., 1969; Joyce and Grindley, 1983). The C-terminal domain (Klenow fragment) contains 5’→3’ polymarase and 3’→5’ exonuclease (editing function) and N-terminal domain possesses 5’→3’ exonuclease activity that removes RNA primers from Okazaki fragments. In the absence of exogenous DNA damage, processing of Okazaki fragments during lagging strand synthesis is probably its main function. It has been observed that the polA1 mutation of E. coli, which is deficient in the Klenow domain but maintains the 5’→3’ exonuclease domain (Joyce et al., 1985), showed a marked increase in deletion and minus frameshift mutations (Fix et al., 1987; Agemizu et al., 1998). Nagata et al (2002) argued that a mismatch bulge formed in the template DNA during lagging strand synthesis (which can lead to sequence shortening in the nascent DNA) can be solved by the 3’→5’ exonulcease activity of Klenow.
Models proposed that detail the mechanisms of deletion mutation fall into two broad classes. The first is based on recombination (break and reunion). In this case, a direct repeat is misaligned in a process that is dependent on sequence similarity. Consistent with this notion is the finding that the deletion frequency was elevated in strains that are hyper-recombinogenic (Coukell and Yanofsky, 1970; Konrad, 1977). The role of recA in deletion formation, however, remains unclear (Franklin, 1967; Albertini et al., 1982; Uematsu et al., 1999). The second model involves replication machinery errors, where the tip of a growing DNA chain is thought to dissociate from one repeat sequence, anneal to another, and then serve as a primer for further DNA synthesis (Albertini et al., 1982; Egner and Berg, 1981). This represents an adaptation of the slipped mispairing model to explain frameshift mutations (Streisinger et al., 1966). Strong support for the replication slip model stems from a study of imprecise excision of the transposon Tn10 in E. coli, which showed that density-labeled parental DNA was not transferred to the progeny during this process (d’Alencon, 1994). DNA polymerase III holoenzyme replication slippage was also detected in vitro on a single-stranded DNA template in a primer extension assay (Canceill and Ehrlich, 1996).
Since the polA1 strain is unable to repair deletion mismatches formed during replication slip, strand bias in spontaneous deletions should be clearly detectable if strandness exists. Using chromosomal genes, the presence of strandness for spontaneous base substitutions and frameshifts has been reported, but not yet for deletions. The results, however, suggested that DNA replication errors for deletions on the E. coli chromosome occur with equal or similar probability on the leading and lagging strands.
E. coli K12 strains KK1 (Wang et al., 1996) and KK300 (this study) represent the polA+ strains used. In KK1, the tonB gene is in the original orientation and the direction of transcription and DNA replication is the same. We refer to this gene as tonBF. KK300 was constructed by inserting a 3108-bp DNA fragment containing the gene in reverse orientation at the tonB site in the chromosome of KK1. Thus, the direction of gene transcription in KK300 is opposite to that of DNA replication. We refer to this gene as tonBR. KK1 and KK300 carry the chloramphenicol-resistance (CmR) gene about 1.9 kb and 4 kb upstream, respectively, of the tonB gene on the chromosome. The CmR gene was used as a selective marker to clone mutant tonB alleles (Kitamura et al., 1995, see Fig. 1). P1 transduction was performed to transfer the polA1 zig219::Tn10 allele (Nagata et al., 2002) into KK1 (tonBF) and KK300 (tonBR), selecting for a tetracycline (Tet)-resistant and ultraviolet-sensitive phenotype, and resulted in KK101 (tonBF polA1, Nagata et al., 2002) and KK301 (tonBR polA1, this study). JC7623 (recBC sbcB) (Winans et al., 1985), JC7623 CmR (Kitamura et al., 1995) and JC7623 tonB::KmR (this study) were used during the construction of KK300. Colicinogenic E. coli CA18 carrying a colicin B factor was used for the preparation of colicin plates (Ishii and Kondo, 1972).
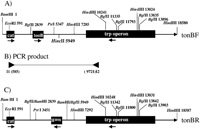 View Details | Fig. 1. Physical map of min 28 of E. coli linkage map tonB-trp (Kitamura et al., 1995). Arrows below solid boxes represent the direction of transcription. Thin lines represent chromosome sequences with the direction of replication indicated by the terminal arrows. A) Physical map of tonBF configuration. An approximately 1.0-kb HindIII-EcoRV fragment 1.6 kb upstream from tonB was replaced with an approximately 1.5-kb fragment containing the cat gene and a new restriction site. Relevant restriction sites with nucleotide numbering of the 18586-bp DNA sequence in which 1 is the first G of the GATC BamHI site are included. In this numbering scheme, the cat sequence was from nt 377 to 1036, tonBF from nt 2993 to 3727 and trp from nt 14864 to 8334. B) Primers used to confirm chromosomal tonBF and tonBR orientations were; I1, 5’-ATCCGGAATTCCGTATGGCAATGAAAGACGGTGAG-3’ (bp 585-617) and I2, 5’-AGCGGATGATTGGCGAAGAAACCAAAGCGCAGATT-3’ (bp 9721-9687). C) Physical map of tonBR configuration. Restriction enzyme-digested fragments at 2838 BglII and 5946 BamHI were reverse-ligated to create the tonBR configuration. In this numbering scheme, the cat sequence was from nt 377 to 1036, tonBR from nt 5065 to 5799 and trp from nt 14864 to 8334. |
Solid and liquid media (both Luria-Bertani broth and M56 minimal media) used were as previously described (Tanaka et al., 2001). Antibiotics were added, if necessary, as follows; Cm, 30 μg/ml; Kanamycin (Km), 50 μg/ml; Ampicillin (Ap), 50 μg/ml; Tet, 10 μg/ml. Colicin plates used were as previously described (Kitamura et al., 1995).
Strains containing the tonB gene in two orientations at the tonB site on the bacterial chromosome were constructed as follows. Plasmid pB0 was used as the starting material for the construction which consisted of a 7284-bp fragment derived from BamHI at nucleotide number 1 to HindIII at nucleotide 7284 containing the cat gene and the tonB gene as shown in Fig. 1A. This fragment was then cloned into the BamHI and HindIII sites of pTZ18R, and the BamHI site disrupted using a Klenow reaction. Plasmid pB0 (CmR tonB+) was partially digested with HincII and then ligated with a BamHI linker at one of the 5 HincII sites. One construct was obtained that possessed the BamHI linker at the HincII site at nucleotide 5946 (see Fig. 1A), named pB0B4. The cat gene on pB0B4 was then disrupted reaction at the EcoRI site using a Klenow, resulting in pB0B4 CmS tonB+. The tonB gene between BglII at nucleotide 2838 and the newly formed BamHI at nucleotide 5946 was then replaced with a KmR gene derived from pKY1292 (Saito et al., 1997), yielding ptonB::Km. Strain JC7623 CmR was transformed with ptonB::Km, which had been linearized following digestion with salI and selected for transformants using KmR and CmS, resulting in JC7623 tonB::KmR. P1 transduction was performed to move the CmS tonB::KmR allele into KK1, and this strain was named KK1 tonB::KmR.
Plasmid pB0B4, which is CmR tonB+, was once again digested with BglII at 2838 and BamHI at 5946, treated with T4 ligase, and a construct possessing the tonB gene ligated in reverse was selected, named pB0B4R. The orientation of tonB was confirmed by verifying the position of the PstI restriction site (Fig. 1). JC7623 tonB::Km was transformed with linearized pB0B4R, carrying CmR tonBR, and selected for CmR KmS tonB+, resulting in JC7623 CmR tonB+. P1 lysate was made from the strain and used to transduce the CmR KmS tonBR allele into strain KK1 CmS tonB::KmR. Selection for CmR KmS tonB+ resulted in KK300. The expected chromosomal orientation was confirmed by PCR as shown in Fig. 2 and by DNA sequencing.
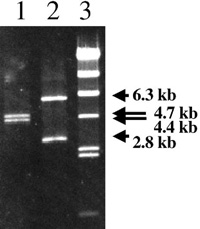 View Details | Fig. 2. Agarose gel separation of PCR-PstI digestion products. PCR amplification from the tonBF chromosome produces 4.4-kb and 4.7-kb fragments following PstI digestion (lane 1), while PCR amplification from tonBR produces 2.8-kb and 6.3-kb fragments following PstI digestion (lane 2). Lambda HindIII markers are in lane 3. |
Mutation to colicin B- and T1 phage- resistance (ColBR) was determined for 10 independent cultures of KK101 (tonBF polA1) and KK301 (tonBR polA1) in 3 ml of L broth following overnight growth. For assays scoring ColBR, independent cultures were directly plated onto colicin plates. ColBR colonies were scored following 48 h incubation at 37°C. Total viable cell counts were determined by serial dilution with phosphate buffer, followed by plating on L agar. Mutation rates, expressed as mutations per cell per generation, were calculated by the method of median (Lea and Coulson, 1949) using the following formula; mutation rate = M/N, where M is the calculated number of mutation events and N is the mean number of viable cells in the culture. M is solved by interpolation from an experimental determination of ro, the median number of ColBr cells determined among the cultures, using the formula ro = M(1.24 + lnM).
For the tonB mutation assay, independent colonies of the strains were inoculated in 3 ml of L broth at 37°C overnight, and 100-μl aliquots were then plated on colicin plates to obtain several hundred mutant colonies per plate. To collect tonB mutants, only one ColBR colony was chosen from each colicin plate, an approach that ensured each mutant analyzed was of independent origin. The DNA fragment containing the mutant tonB gene was amplified by PCR using appropriate primers from genomic DNA that had been extracted from the tonB mutant. Following amplification, the concentration of amplified DNA was determined by examination of band intensities following electrophoresis of 1-μl samples through 0.7% agarose gels. Mutant sequences were determined by the dideoxy chain termination method using an automated sequencer.
The chi-square test was used to examine differences in mutation between tonBF and tonBR. P<0.05 was regarded as significant.
In an effort to investigate the possibility of differences in replication fidelity between leading and lagging strand DNA synthesis, we developed a system to measure mutations in a pair of chromosomal tonB target genes as mentioned in the MATERIALS AND METHODS and as illustrated in Fig. 1. When the gene possesses its original orientation (referred to as tonBF), the direction of gene transcription and replication fork movement is identical. Thus, the transcribed strand is the template for leading strand synthesis. On the other hand, in the reverse orientation (tonBR), the direction of gene transcription and replication fork movement is opposite. Thus, the transcribed strand is the template for lagging strand synthesis. The presence of the respective two genes in opposite orientation was verified by PCR-PstI polymorphism (Fig. 2). The 9.1-kb PCR fragment (Fig 1B) was digested into 4.4-kb and 4.7-kb fragments by Pst I at 5342 in the case when the gene was tonBF, and 6.3-kb and 2.8-kb fragments by Pst I at 3446 in the case when the gene was tonBR.
The system is based on a comparison of the mutability of the tonB gene, selected as ColBR, when present in two opposite orientations on the chromosome relative to the advancing replication fork. The ColBR mutants were DNA-sequenced to obtain the entire spectra of mutations. Thus, a comparison of the mutability of the same target gene in two orientations at the same position on the chromosome can reveal a difference in mutability, if any, of leading versus lagging-strand mutagenesis.
Using this system, we first measured the spontaneous mutation rate of the pair KK1 (tonBF poAl+) and KK300 (tonBR polA+), and the pair KK101 (tonBF polA1) and KK301 (tonBR polA1), which were selected as having the ColBR phenotype. The ColBR mutation rate of KK300 was 2.61 × 10–8, which did not differ significantly from that of KK1 (1.30 × 10–8). The ColBR mutation rate of KK301 was 10.87 × 10–8, which was higher than that observed in KK1 and KK300, but not significantly different from the rate in KK101 (8.78 × 10–8, Table 1).
 View Details | Table 1. Spontaneous endogenous tonB mutations in a pair of polA1 strains containing the tonB gene in opposite (F and R) orientation on the chromosome |
The mutant sequences in 114 KK101 (tonBF polA1) and 76 KK301 (tonBR polA1) clones were determined and the distribution by class is listed in Table 1. The most frequent mutational event in the KK101 (tonBF polA1) strain was represented by deletions followed by frameshifts and base substitutions (Table 1). These spectra were similar to those of KK301 (tonBR polA1) where deletions predominated, followed by frameshifts. In the polA1 pair, the difference in base substitutions, deletions and frameshifts between the forward and reverse direction was not significant. Caution is required, however, when drawing conclusions since the current sample size of mutations analyzed may be statistically insufficient to address the issue of strandedness in detail.
Table 2 shows the 33 deletions identified from KK301 (tonBR polA1), among which 15 were at different sites, and ranged in size from 4 to 11794-bp. Nine of these sites were flanked by repeats (Table 2 bold letters) of two or more bases, suggesting both the presence of direct repeats for deletion formation and the absence of direct repeats for deletion formation (Uematsu et al., 1999). These features consisting of deletions in KK301 (tonBR polA1) were similar to those obtained from the 58 deletion mutations at 24 different sites in KK101 (tonBF polA1), in which 17 of the 24 sites were flanked by repeating sequences. Among the deletions in the polA1 strain, one in particular (13 bp with 3-bp repeats, nucleotides 3023–3035) accounted for 18 of the 33 deletions in polA1 tonBR and 31 of the 58 deletions in polA1 tonBF. Thus, replication slippage occurs equally during leading strand synthesis and lagging strand synthesis at this particular site. We therefore argue in general that deletions occur with similar probability during leading and lagging strand synthesis.
 View Details | Table 2. Location and type of deletion mutations in polA1 strains with the tonB gene in opposite orientation. |
By constructing a pair of E. coli strains possessing the endogenous target gene tonB in opposite orientation with regard to oriC (Fig. 1), we set to determine whether the two DNA strands are replicated with similar accuracy during chromosomal replication in the pair of polA1 strains. Previously, Nagata et al (2002) argued that DNA polymerase I, encoded by the polA gene, plays a role in recognizing and correcting deletion, duplication and frameshift mismatches resulting from DNA polymerase III holoenzyme replication errors. In this case, duplications and plus frameshifts are elevated in the polA107 mutation, defective for 5’→3’ exonucease activity (Joyce et al., 1985), and deletions and minus frameshifts are elevated in the polA1 mutation, defective for Klenow activity (Joyce et al., 1985). Thus, 5’→3’ exonuclease activity can repair replication bulges in the nascent strand (which can lead to plus frameshifts or duplication) and the Klenow 3’→5’ exonuclease activity can solve replication bulges in the template strand (which can lead to deletions or minus frameshifts). It has been argued that polymerase III replication error bulges occur preferentially in lagging strand synthesis (Rosche et al., 1995). In the absence of DNA damage, processing of Okazaki fragments during lagging strand synthesis remains the function of DNA polymerase I. Thus, it is reasonable to assume that replication-dependent deletion may occur preferentially in lagging strand synthesis. Since the polA1 strain is unable to process replication bulges thus formed, the strain can be a deletion mutator. Our results in this study suggest that spontaneous mutagenesis for deletions occurred with similar likelihood on the leading and lagging strands. This conclusion stems from the observations that 1) Mutation frequencies in the KK101 (tonBF polA1) and KK301 (tonBR polA1) pair did not differ (Table 1) and 2) specific deletion hotspots in polA1 strains were equally observed in KK101 (tonBF polA1) and KK301 (tonBR polA1) (Table 2). Thus, we argue that there is no difference in the fidelity for deletion formation during chromosomal replication between leading strand synthesis and lagging strand synthesis.
As mentioned above, DNA polymerase I can excise oligonucleotides and fill gap thus formed during DNA repair (Kornberg and Baker, 1992). Since we used polA1 strain, other DNA polymerases can replace DNA polymerase I during endogenous damage repair which can occur both leading strand and lagging strand equally. We therefore assume that deletions in this study may occur during repair synthesis stage.
The observations made here differ from those made by Trinh and Sinden (1991) and Iwaki et al. (1996) where it was reported that spontaneous deletions and minus frameshifts, respectively, in E. coli occurred preferentially during lagging strand synthesis. Yoshiyama et al. (2001) also observed a strong directionality in the movement of the replication fork during formation of spontaneous minus frameshifts. These observations were made using target genes on plasmids. The ColE1 plasmids used in these experiments replicate starting from the origin with synthesis by DNA polymerase I (Marians, 1992), rather than by DNA polymerase III, which complicates any comparisons.
In the case of damage-induced mutagenesis, a preference for the lagging strand by AAF has been argued (Veaute and Fuchs, 1993). On the other hand, no preference for either strand was reported for the induction of G:C→T:A transversion mutations by 8-oxoguanine (8-oxoG) (Wagner et al., 1997; Watanabe et al., 2001). These experiments were conducted using ColE1 type plasmids. Reed and Hutchinson (1987) observed no preference for the leading or lagging strand in the induction of G:C→A:T transition mutations by nitrosoguanidine (NG). In that case, the target gene was lysogenic λCI, which is on the chromosome. Differences between AAF lesions and 8-oxoG or NG lesions exist in their capacity to interfere with DNA polymerase. AAF adducts have been shown to block DNA replication (Belguise-Valladier et al., 1994), whereas NG lesions (Loechler et al. 1984) and 8-oxoG (Wood et al., 1990) permit efficient translesion synthesis. Thus, it has been assumed that mutational strand bias is correlated with blockage of the replication machinery. UV-induced DNA lesions, mainly pyrimidine dimers and 6–4 adducts, can act as blocks to DNA replication (Borden et al., 2002). Gawel et al. (2002b) demonstrated no strand bias in UV lesion translesion DNA synthesis using a lacZ reversion assay. However, Armstrong and Kunz (1995) demonstrated a bias in UV-induced mutagenesis using a Saccharomyces cerevisiae-plasmid system. Thus, the correlation between strand bias and blockage of the replication machinery remains unclear.
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan and from the Japan Society for the Promotion of Science. YN is the recipient of a postdoctoral fellowship from the Japan Society for the Promotion of Science (07221).