| Edited by Kazuhiro Kutsukake. Masahiro Ishiura: Corresponding author. E-mail: ishiura@gene.nagoya-u.ac.jp. Kazuhiro Aoki: Present address: Department of Tumor Virology, Research Institute for Microbial Diseases, Osaka University, Yamadaoka, Suita-shi, Osaka 565-0871, Japan |
Circadian rhythm is a self-sustaining oscillation whose period length coincides with the 24-hour day-night cycle. Many biological activities show circadian patterns, allowing organisms to adapt to daily fluctuations in the environment. Circadian rhythms are widespread and involved in functions as diverse as human sleep-wake cycles and cyanobacterial nitrogen fixation. Endogenous oscillators that generate circadian rhythms are called circadian clocks. Cyanobacteria are the only bacterial species found to have circadian clock. Three clock genes — kaiA, kaiB, and kaiC — have been identified in Synechococcus sp. strain PCC 7942, and positive and negative feedback regulations of the kai genes are proposed to be the core mechanism generating the circadian oscillation (Ishiura et al., 1998).
Real-time automated bioluminescence monitoring is a powerful tool for circadian clock research since it allows high resolution, sustained measurements of circadian rhythms (Aoki et al., 1995; Kondo et al., 1993; Millar et al., 1992; Onai et al., 2004). In the case of cyanobacteria, a promoter region of a clock-controlled gene is fused to bacterial luciferase gene luxAB, and the resulting fusion gene is integrated into the genome (Aoki et al., 1995; Kondo et al., 1993; Onai et al., 2004). Then, rhythmic regulation of the promoter activity is continuously monitored as bioluminescence using n-decanal, a membrane-permeating and volatile substrate without perturbing cells.
We previously demonstrated bioluminescence circadian rhythms in the cyanobacterium Synechocystis sp. strain PCC 6803 using a strain CFC2, which carries a luxAB bioluminescence reporter gene fused to a promoter region of the dnaK gene (PdnaK) (Aoki et al., 1995). Because Synechocystis can carry out light-activated heterotrophic growth in glucose-supplemented medium in darkness (Anderson and McIntosh, 1991), the system is useful for elucidating the circadian regulatory mechanisms in darkness (Aoki et al., 1997). In addition, by performing DNA microarray analysis of clock-controlled genes, we identified many interesting genes implicated in circadian clock output mechanisms (Kucho et al., 2004). Despite very high activity of dnaK promoter, however, bioluminescence intensity in strain CFC2 is low (approximately 1/20th that of the psbAI reporter strain of Synechococcus) (Aoki et al., 1995). This hampered further application of the bioluminescence reporter system to genes with weak promoter activity. Strain CFC2 had two known problems (Fig. 1A): (i) the 102-bp sequence that was inserted at the junction of the PdnaK and luxA coding sequences added 34 extra amino acids to the amino-terminus of the LuxA protein, and (ii) the reporter construct interrupted the coding sequence of the ssl0410 gene. It seemed likely that the strain’s low bioluminescence intensity was a result of these factors, especially factor (i). In the present study, we report a great improvement in the bioluminescence intensity in Synechocystis.
 View Details | Fig. 1. Schematic representation of the bioluminescence reporter constructs and the integration sites in the Synechocystis genome. (A) pCF5, which was used to generate strain CFC2 (Aoki et al., 1995). (B) pTS1PdnaK::luxAB(–) and pTS1PdnaK::luxAB(+), which were used to generate strains PdnaK::luxAB(–) and PdnaK::luxAB(+), respectively. The boxes with an arrowhead indicate open reading frames (ORFs) and their transcription direction. Arrowheads designate PCR primers used for confirmation of the expected integration of the reporter constructs. ORF numbers are according to the CyanoBase (http://www.kazusa.or.jp/cyano/). |
A glucose-tolerant strain of Synechocystis sp. strain PCC 6803 was maintained in BG-11 liquid medium (Rippka et al., 1979) or on BG-11 agar medium containing 1 mM sodium thiosulfate, 10 mM N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (TES, pH 8.2), and 1.5% Bacto Agar (Nippon BD, Tokyo, Japan) at 30°C under constant light (LL) from white fluorescent lamps at 67 μmol m–2 sec–1. Transformants were selected and segregated on BG-11 agar medium or BG-11 liquid medium containing 40 μg/ml spectinomycin sulfate. Escherichia coli strains HB101 and DH5α were maintained at 37°C in Luria-Bertani broth (LB) liquid medium or on LB agar medium containing 1.2% agar (Sambrook et al., 2001). DNA manipulation and sequ-encing were performed by standard methods (Sambrook et al., 2001).
To generate a PdnaK::luxAB fusion gene, we amplified a dnaK promoter segment (nucleotides –1 to –805, with the A of the ATG translation initiation codon defined as +1) by PCR using Synechocystis genomic DNA as a template and the primer set PdnaK805F (5’-TTCTTAAGGTGACCATCAAGCCGGTGCG-3’; the AflII site is underlined) and PdnaK805R (5’-TTCATATGTTTAATTAACTCCCGTTAAC-3’; the NdeI site is underlined, and the CAT sequence complementary to the ATG initiation codon of the dnaK gene is italicized). The amplified fragment was subcloned into pT7Blue-T (Novagen, Madison, WI, USA) to yield pT7PdnaK. We amplified a 2.1-kb fragment carrying the coding region of the Vibrio harveyi luxAB gene set (Baldwin et al., 1984) by PCR with plasmid pLAV1 (gift of T. O. Baldwin) as the template and the primer set Lux-F1M (5’-TCATATGAAATTTGGAAACTTCCTTC-3’; the NdeI site is underlined, and the ATG initiation codon of the luxA gene is italicized) and Lux-R1M (5’-TTCTAGATTACGAGTGGTATTTGACG-3’; the XbaI site is underlined), and subcloned into pT7Blue-T to yield pT7Blue-T/luxAB. We excised the luxAB gene segment from pT7Blue-T/luxAB as a 2.1-kb NdeI-XbaI fragment and ligated it into the NdeI-XbaI site of pT7PdnaK, which is just downstream of PdnaK, yielding pT7PdnaK::luxAB.
To generate a targeting vector that allows integration of the PdnaK::luxAB fusion gene into the Synechocystis genome (targeting site 1) by homologous recombination, we amplified 0.5-kb and 0.7-kb segments that corresponded to the genomic regions of Synechocystis between positions 2,370,160 and 2,370,681 and between 2,370,682 and 2,371,369 (Kaneko et al., 1996) by PCR using primer sets Up68NS1U (5’-CAGCTGTCCCAGCCTCTCAACCAC-3’) and Up68NS1L (5’-AGATCTGAATAGAAGAGCGAT-AAT-3’; the BglII site is underlined), and Lo68NS1U (5’-AGATCTAGTGTAGGCGGTAAAGTC-3’; the BglII site is underlined) and Lo68NS1L (5’-CAGCTGTTGGTGGAAA-GTTGGCTC-3’), respectively. The amplified fragments were digested with BglII and ligated into pGEM-T (Promega, WI, USA) to yield pUL68TS1. For elimination of a NdeI site of pUL68TS1, the plasmid was digested with NdeI, blunt-ended with the Klenow fragment, and self-ligated. The resulting plasmid, pUL68TS1ΔNde, was digested with BglII and ligated with a double-stranded oligonucleotide containing BamHI, AflII, KpnI, and EcoRV restriction sites (d[pGATCGGATCCCTTAA-GGGTACCGATATC]) to yield pUL68TS1ΔNdeMCS. We inserted the spectinomycin resistance gene (Spr) (Prentki and Krisch, 1984) and the transcription terminator sequence of the E. coli rrnB gene (Orosz et al., 1991) derived from pTrc99A (GenBank accession number M22744) into the BamHI and KpnI sites of pUL68-TS1ΔNdeMCS, respectively, yielding pUL68TS1ΔNde-MCSΩT.
We excised the PdnaK::luxAB fusion gene from pT7PdnaK::luxAB as an AflII-XbaI fragment and ligated it into the AflII-XbaI site of pUL68TS1ΔNdeMCSΩT, which locates between Spr and the rrnB transcription terminator, to generate pTS1PdnaK::luxAB(–) (Fig. 1B). To generate pTS1PdnaK::luxAB(+), we excised a Spr-containing fragment from pTS1PdnaK::luxAB(–) by BamHI digestion and ligated it back into the same BamHI site. We selected a clone in which Spr and the PdnaK ::luxAB fusion gene were transcribed in opposite directions (Fig. 1B). We sequenced all the PCR-amplified fragments and all the junctions resulting from ligation reactions and confirmed that their nucleotide sequences were correct.
We transferred the DNA constructs into Synechocystis cells as previously described (Williams, 1988) and selected spectinomycin-resistant colonies. We confirmed by PCR that the reporter constructs were integrated into targeting site 1 using the genomic DNA as a template and the primer set p-ts1-1 (5’-TGGCTTTGGGCGGGAACTTG-3’) and p-ts1-2 (5’-GCCATATTTAACGGGACAGC-3’) (Fig. 1B).
Synechocystis cells were grown on BG-11 agar under LL at 30°C for 3 days and then subjected to 12 h of darkness, which synchronized the circadian clock. Bioluminescence from the cells was then automatically monitored under LL (47 μmol m–2 sec–1) with n-decanal as the substrate using the photomultiplier tube-based bioluminescence monitoring system as described previously (Aoki et al., 1995). Bioluminescence intensity was measured for 10 sec in darkness at 38 min intervals.
To address problems in Synechocystis bioluminescence reporter strain CFC2, we made a new reporter construct, pTS1PdnaK::luxAB(–) (Fig. 1B) in which we seamlessly fused PdnaK to luxA so that the initiation codon of the luxA gene was located at the same position as it was in the native dnaK gene. In this construct, 34 extra amino acids that were attached to the amino-terminus of the LuxA protein expressed in strain CFC2 were removed. Removal of this extra sequence would enhance the LuxA activity. The fusion gene was integrated into an intergenic site in the genome where two genes, slr0370 and sll0337, are “tail-to-tail” (targeting site 1). Since integration of exogenous DNA fragments at this site disrupts neither the coding nor the promoter sequences of the two genes, secondary effects due to their disruption were avoided. In addition, we used a short, 805-bp PdnaK seg-ment that conferred similar rhythmic expression to the 2.2-kbp PdnaK segment used in strain CFC2 (Aoki et al., 1995). We transferred pTS1PdnaK::luxAB(–) into Synechocystis cells and assayed the resulting strain, PdnaK ::luxAB(–), for bioluminescence rhythm. The strain showed a robust circadian oscillation that was similar in period length and phase to the oscillation of strain CFC2 (Fig. 2). Amplitude of the oscillation was 11% higher than that of strain CFC2. The mean bioluminescence intensity over 168 h was 12 times that of strain CFC2 (Fig. 3).
 View Details | Fig. 2. Bioluminescence rhythms of the reporter strains. Synechocystis cells were subjected to 12 h of darkness, which synchronized the circadian clock, and were then transferred to LL for monitoring of bioluminescence rhythms. The light regimens are shown by filled (darkness) and open (LL) boxes above the graph. Vertical axis represents the bioluminescence intensity normalized by the number of colonies. Horizontal axis represents time after transfer to LL. Bioluminescence intensity in strain CFC2 is different from that appeared in Aoki et al. (1995) because unit of intensity and bioluminescence monitoring apparatus were different. |
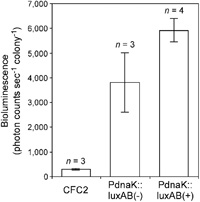 View Details | Fig. 3. Bioluminescence intensity of the reporter strains. Boxes represent mean bioluminescence intensity over 168 h for the indicated number (n) of experiments, normalized by the number of colonies. Bars represent the SD. |
Next, we tested the effects of transcription direction of the selection-marker Spr gene on bioluminescence intensity. In strain PdnaK::luxAB(–), Spr was located upst-ream of the PdnaK::luxAB fusion gene and transcribed in the same direction (Fig. 1B). We generated a reporter construct, pTS1PdnaK::luxAB(+), in which transcription of the two genes occurred in opposite directions, and integrated it into the same locus as pTS1PdnaK::luxAB(–) (Fig. 1B). Fig. 2 shows the bioluminescence rhythm of the resulting strain PdnaK::luxAB(+). This strain’s rhythmic pattern was similar to that of strain PdnaK ::luxAB(–), but the mean bioluminescence intensity was 60% greater than that of strain PdnaK::luxAB(–) (Fig. 3). Amplitude of the rhythm was 10% higher than that of strain CFC2. In strain PdnaK::luxAB(–), movement of RNA polymerase on Spr toward the PdnaK::luxAB fusion gene would cause positive supercoiling of dnaK promoter region. This would negatively affect transcription of the PdnaK::luxAB. Finally, the bioluminescence intensity of strain PdnaK::luxAB(+) was 19 times that of strain CFC2 while the period length and phase of the rhythm were unaffected. As a result, the improved bioluminescence intensity of strain PdnaK::luxAB(+) was comparable with that of the Synechococcus psbAI reporter strain (Kondo et al., 1993).
In this study, we elucidated the critical factors for generating reporter strains with high bioluminescence intensity: (i) seamless fusion of the PdnaK sequence to the luxA gene, and (ii) anti-parallel positioning of the fusion gene and the Spr selection marker gene. These will be useful for analyzing bioluminescence rhythms of genes with low promoter activity. Furthermore, the new constructs should facilitate analysis of a large number of promoters because the promoter fragment contained in the constructs can easily be replaced by digestion with unique AflII-NdeI sites (Fig. 1B). Recently, we developed a high-throughput bioluminescence monitoring apparatus using 96-well microplates (Okamoto et al., 2004). Thus, the new reporter constructs should make a significant contribution to genome-wide analysis of circadian gene expression in Synechocystis.
We thank Dr. Thomas O. Baldwin (Texas A&M University) for the gift of plasmid pLAV1, and Dr. Miriam Bloom (SciWrite Biomedical Writing & Editing Services) for professional editing. This work was supported by grants from the Japanese Ministry of Education, Science and Culture (MEXT), ‘Program for Promotion of Basic Research Activities for Innovative Biosciences’ (PROBRAIN) promoted by BRAIN, ‘Research for the Future Novel Gene Function Involved in Higher-Order Regulation of Nutrition-Storage in Plants’ promoted by the Japan Society for the Promotion of Science, ‘Ground-based Research Announcement for Space Utilization’ promoted by the Japan Space Forum, ‘National Project on Protein Structural and Functional Analyses’ promoted by MEXT, and ‘Joint-Project for Leading Science and Technology’ promoted by the Aichi Science and Technology Foundation to M.I. Division of Biological Science, Graduate School of Science, Nagoya University was supported by a 21st century COE grant from MEXT.
Added in proofs:
We developed an integrated bioluminescence rhythm-analyzing program (RAP) with a user-friendly graphical interface [Okamoto, K., Onai, K., and Ishiura, M (2005) RAP, an integrated program for monitoring bioluminescence and analyzing circadian rhythms in real time. Anal Biochem. in press].
|