| Edited by Kiyotaka Okada. Shigeyuki Kawano: Corresponding author. E-mail: kawano@k.u-tokyo.ac.jp Accession Numbers: The SlMDL1, SlSs, SlGh17, SlAPG and SlChs sequences were submitted to GenBank under accession numbers AB182105, AB182104, AB182102, AB182103, and AB182106, respectively. |
Silene latifolia has two morphologically distinguishable sex chromosomes, X and Y. Sex determination in this plant is controlled by the Y chromosome, which encodes male-determining factors. The male plant (22A + X + Y) develops staminate flowers with ten stamens and a rudimentary pistil, whereas the female plant (22A + 2X) has pistillate flowers bearing styles, a superior ovary, and ten rudimentary stamens (Matsunaga and Kawano, 2001; Negrutiu et al., 2001). Two main developmental processes that occur in the S. latifolia floral bud, i.e., the arrest of pistil development and the promotion of stamen development, both occur in the male plant. In the female plant, the pistil develops due to the absence of the Y chromosome (Westergaard, 1958).
Smut fungi are plant-parasitic basidiomycetes that produce smut spores termed teliospores (Martinez-Espinoza et al., 2002; Vánky, 1998). Following the systemic infection by the parasitic smut fungus Ustilago violacea (Microbotryum violaceum), female S. latifolia plants develop stamens, and their rudimentary stamens develop into anthers (Uchida et al., 2003). These induced anthers contain teliospores instead of pollen. They contain little or no tapetal tissue, which is important for the development and maturation of pollen (Audran and Batcho, 1981). As a result, the ovules of the infected flowers are sterile and reduced in size (Young, 2002).
Anther development in both healthy and infected flowers initiates with the emergence of the stamen primordia in stage 6. Throughout stages 8–11, within the stamen primordia in healthy males, cell specification and differentiation events direct the morphology of anthers, the differentiation of pollen mother cells, the entrance of the pollen mother cells into meiosis to generate tetrads, and the formation of mature pollen grains. The sporogenous cells in infected females remain unspecialized and are destroyed during these stages (Uchida et al., 2003).
The parasitic smut fungus can infect both male and female flower producing the same symptoms on both flowers. Interestingly, some of the infected male plants retained partial fertility, and can produce fertile pollen grains. By contrast, no fertile pollen was observed in any flower stalks in the infected female plants. This is probably because only the male genome contains the Y chromosome with its complete sets of anther formation and maturation genes (Scutt et al., 1997; Uchida et al., 2003).
In this work, a cDNA subtraction method using healthy S. latifolia male buds and female buds infected by M. violaceum was carried out to isolate genes that are upregulated in the male flower in the late stages of anther development. We isolated and characterized five genes that are expressed in male S. latifolia buds. The isolated genes showed high expression in male flower buds. Interestingly, low or no expression of the isolated genes was identified on either healthy or infected female flower buds. These cDNAs are likely directly related to anther development and pollen maturation, and should be under the control of the male fertility factors normally encoded on the Y chromosome. The corresponding genes are expressed between meiosis and microspore mitosis, a timing that corresponds to the burst of tapetal activity in the developing anther.
Seeds of Silene latifolia (Miller) E.H.L. Krause were kindly provided by Dr. Sarah Grant of the University of North Carolina. The seeds were pre-cultured in the dark in tap water at 4°C for at least 4 days and then cultured at 23°C. Long-day conditions (illumination for 16 h per day) allowed the plants to flower. Harvested plant tissues were frozen in liquid nitrogen and stored at –80°C until use for the isolation of DNA and RNA. The haploid Microbotryum violaceum (Pers.) G. Deml and Oberwinkler strains ATCC-22000 and ATCC-22004 were grown and used to infect male and female S. latifolia plants as previously described (Uchida et al., 2003).
Total RNA was isolated from both male and infected female buds using TRIzol reagent (Gibco BRL, Grand Island, NY). mRNA was isolated from the total RNA using the PolyA Tract mRNA isolation System III (Promega, Madison, WI). A subtraction library was generated using the Clontech PCR-Select cDNA Subtraction Kit (Clontech, Palo Alto, CA). mRNA from healthy male buds was used as the “tester,” and mRNA from the fungus-infected female buds was used as the “driver”. After two successive rounds of subtraction, the subtracted cDNAs were enriched by PCR and fused into the pCR 2.1-TOPO cloning vector using the TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA).
Virtual Northern blots (Franz et al., 1999), which are Southern blots of PCR-amplified cDNA samples, were used to confirm the male specificity of the isolated subtracted products. Using mRNA from healthy male buds, healthy female buds, infected male buds , infected female buds, roots, and leaves as starting materials, first-strand cDNAs were prepared using the Super SMART PCR cDNA Synthesis kit (Clontech) such that PCR primer sites were incorporated at both ends of the cDNAs. The products were then used in PCR amplifications of the entire cDNA populations. Amplified cDNA samples were concentrated by ethanol precipitation, resuspended in alkaline loading buffer, and analyzed on 1.5% alkaline agarose gels (8 μg per lane) together with DNA size standards. The alkaline agarose gels were neutralized, and the DNAs were blotted onto Immobilon-Ny+ membrane (Millipore, Bedford, MA) as described (Sambrook et al., 1989). Labeling and detection of probes were performed using the Gene Images AlkPhos Direct Labeling and Detection System (Amersham Biosciences, Piscataway, NJ).
Genomic DNA was extracted from S. latifolia leaves using the Nucleon PhytoPure Genomic DNA Extraction Kit (Amersham Biosciences). Genomic DNA samples were digested with HindIII restriction enzymes and analyzed on 1% agarose gels prior to denaturation and capillary blotting onto Immobilon-Ny+ membrane as described (Sambrook et al., 1989). Subtracted cDNA clones were digested by HindIII and used for the detection of the copy number. Probe labeling and detection were performed as for the virtual Northern blots.
In situ hybridization was performed according to Kazama et al., (2005). DNA fragments used for genomic southern were cloned into the pBluescript II SK+ (Stratagen, LaJolla, CA) and used for in vitro transcription of the probes. Sense and antisense probes were synthesized using the DIG RNA Labeling Kit (SP6/T7) (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. After hybridization at 65°C and washing with 0.2 × SSC, the samples were incubated for 10 min in NT buffer (100 mM Tris-HCl [pH 7.5], 150 mM NaCl) and incubated for 60 min in blocking buffer (NT buffer containing 1.5% blocking reagent; Roche Diagnostics). Anti-digoxigenin alkaline-phosphatase-conjugated antibody (Roche Diagnostics) was diluted 1:1000 with blocking buffer, and 400 μl of the dilution were applied to each slide. The slides were incubated over night and then washed three times with NT buffer. The sections were stained with NTM buffer (100 mM Tris-HCl [pH 9.5], 100 mM NaCl, 50 mM MgCl2) containing 450 μg/ml nitroblue tetrazolium (NBT) and 175 μg/ml 5-bromo-4-chloro-3-indolyl-phosphate (BCIP). The staining reaction was stopped with TE buffer (Tris-HCl [pH 8.0], 1 mM EDTA). Photographs were taken with an Olympus BX52 research microscope (Olympus, Tokyo, Japan).
The subtracted clones were sequenced using a Big Dye Terminator Cycle Sequencing FS Ready Reaction Kit (Applied Biosystems, Foster City, CA) and an ABI PRISM 3100 DNA sequencer (Applied Biosystems). Pairs of partial nucleotide sequences with greater than 95% identity over at least 100 bp were considered identical. A homology search was performed using BLASTX against the NCBI protein database (http://www.ncbi.nlm.nih.gov).
Sequences of plant chalcone synthase genes and those of the plant glycosyl hydrolase 17 family that showed similarity to the S. latifolia chalcone synthase (SlChs) and S. latifolia glycosyl hydrolase 17 (SlGh17) genes were obtained from the NCBI non-redundant and dbEST data sets using BLASTX or BLASTP (ver. 2.0.10) (Altschul et al., 1997). The full amino acid sequences of the proteins were aligned using CLUSTAL W ver. 1.8 (Thompson et al., 1994) and subjected to phylogenetic analysis. Phylogenic trees were constructed using the neighbor-joining (NJ) method (Saitou and Nei, 1987) with parsimony and heuristic search criteria and 1000 bootstrap replications to assess branching confidence.
To isolate novel genes responsible for the development and maturation of pollen grains in S. latifolia, we employed a cDNA subtraction between healthy male buds (as a tester) and female buds infected with the smut fungus M. violaceum (as a driver). Buds of 1.0–3.5 mm (stages 8–11, (Farbos et al., 1997)) were selected for subtraction, since a burst of tapetal activity and development of pollen grains occur during these stages. After two successive rounds of subtraction, a subtracted library of 1356 cDNA clones was constructed. To confirm the integrity of the subtracted library, 288 clones were randomly picked and sequenced. The resulted cDNA sequences were subjected to a homology search using BLASTX. Seventy two clones showed no homology with the any protein within the GenBank, 18 clones were ribosomal protein, 11 clone showed similarity with expressed protein with no specific function, and 151 clone showed homology with known genes in organisms other than S. latifolia. Thirty-six clones of the sequenced 288 clones corresponded to previously identified male-specific S. latifolia genes, including men2, men3, men8, men9, men369 (Scutt et al., 1997; Scutt et al., 2002), MROS3A, MROS3B (Matsunaga et al., 1996), ST1 (Lebel-Hardenack et al., 1997), CCLS4 (Barbacar et al., 1997), SlX1 (Delichere et al., 1999), and SLP2 (Pritham et al., 2003).
Instead of infected female buds, healthy female buds were used as a template in differential screening to ensure the male-specificity of the isolated clones. It was not possible to use Northern blots because of the large number of clones and the expected low expression of some of the genes. Therefore, virtual Northern blots were used to analyze cDNAs amplified by PCR from mRNA of healthy male buds and healthy female buds. Although SMART amplification maintains representation of relative gene expression (Seth et al., 2003), we only selected clones showing male specific bands. The constitutively expressed actin gene was used to confirm equal loading (Fig. 1). Based upon the sequence data, the ribosomal genes, cDNA previously isolated in S. latifolia, housekeeping genes and genes expressed in vegetative organs were excluded from the screening of the 288 clones. Five unique cDNA clones showing male-specific expression were identified (Fig. 1). The transcripts of these five clones were detected only in male buds. The transcription levels of the isolated clones were below the limit of detection in roots, leaves, and healthy female buds, confirming the male-specificity of the isolated clones (Fig. 1).
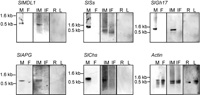 View Details | Fig. 1. Virtual Northern hybridization of the five isolated male-specific cDNA probes derived from cDNA subtraction. The virtual Northern blots contain RT-PCR products derived from S. latifolia male buds (M), female buds (F), fungus-infected male buds (IM), fungus-infected female buds (IF), roots (R), and leaves (L). All of the identified cDNAs show male-specific hybridization bands. Equal loading of the lanes was demonstrated using the expression of the constitutively expressed actin gene. The molecular sizes of prominent hybridization signals are indicated. |
The sequences of the isolated cDNAs were submitted to GenBank and subjected to a homology search using BLASTX. The five cDNA clones were named SlMDL1, SlSs, SlGh17, SlAPG and SlChs (accession numbers AB182105, AB182104, AB182102, AB182103, and AB182106, respectively), as their deduced amino acid sequences showed strong similarities to those of the mandelonitrile lyase family, the strictosidine synthase family, a glycosyl hydrolase family 17 protein, an anther-specific proline-rich protein APG precursor, and the chalcone synthase family, respectively (Table 1).
 View Details | Table 1 cDNAs isolated in this study |
The expression patterns of the identified genes were tested in both infected male and infected female buds. Expression of the isolated genes was monitored only in the infected male flower buds (Fig. 1). No expression of these genes was monitored in the infected female flower, even with more cDNA amplification of the infected female flower bud.
The expression patterns of the identified genes during the development of male flower buds were investigated using virtual Northern blots (Fig. 2). The blots were performed on PCR-amplified cDNA from early male flower buds of 1.0-3.5 mm (stages 8-11, (Farbos et al., 1997)) and late male flower buds of 7.0–15.0 mm (stage 12 until dehiscence). The SlGh17, SlAPG, SlSs, SlMDL1, and SlChs genes showed strong hybridization signals in early male flower buds (Fig. 2). Transcripts of these five genes accumulated preferentially in male flower buds from stages 8 to 11. During these stages, the anthers are characterized by a burst of tapetal activity, callose deposition, and the entrance of pollen mother cells into meiosis to form tetrads (Farbos et al., 1997).
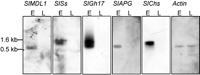 View Details | Fig. 2. Transcripts of the isolated cDNA clones are preferentially expressed in stages associated with a burst in tapetal activity. The virtual Northern blots contain RT-PCR products derived from S. latifolia male buds of 1.0-3.5 mm (E) and healthy male buds of 7-15 mm (L). The genes are preferentially expressed between meiosis and microspore mitosis, a timing that corresponds to a burst in tapetal activity in the developing anther. Equal loading of the lanes was demonstrated using the expression of the constitutively expressed actin gene. The molecular sizes of prominent hybridization signals are indicated. |
Genomic Southern blot analysis was used to determine the distribution of the isolated clones in the male and female S. latifolia genomes and to evaluate their copy numbers. The hybridization profiles are illustrated in Fig. 3, and the copy numbers of each clone are listed in Table 1. None of the signals from any of the clones differed between the male and female DNA samples (Fig. 3), suggesting that none of the five genes is located on the Y chromosome. The SlAPG and SlChs clones showed one and two bands per digest, respectively, indicating that they belong to small copy number gene families. The other isolated clones showed three to five bands per digest, indicating intermediate (3–4) copy numbers.
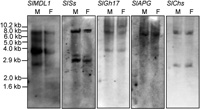 View Details | Fig. 3. Genomic Southern blot analysis of the isolated cDNA clones. Blots of genomic DNA (20 μg per lane, digested with HindIII) were probed with the cDNA clones indicated. Clones with an average of less than three signals per digest were scored as low (SlAPG and SlChs), and those with three to seven bands were scored as intermediate. The data are summarized in Table 1. |
The expression patterns of the SlGh17, SlAPG, SlSs, SlMDL1, and SlChs genes inside the anther of the healthy male flower buds were analyzed by in situ hybridization (Fig. 4). The genes were highly expressed inside the anthers of the male flower buds (Fig. 4). In contrast, there was low to no expression in sepals, petals, or suppressed gynoecia (data not shown). According to the location of expression inside the anther, the five genes could be classified into two groups. The first group, which contains SlMDL1. SlSs, SlGh17, showed expression in both tapetum and pollen grains. Strong accumulation of these genes was monitored first on the tapetum (Fig. 4A, B, and C). At later stages, expression of SlMDL1. SlSs, SlGh17 genes can be identified in the pollen grains (Fig. 4F, G, H, close up in Fig. 4K, L, and M). The second group, which contains SlAPG and SlChs, showed expression only in the tapetum of the male flower buds (Fig. 4D, E, I, and close up N). The degeneration of the tapetum in stage 12 resulted in a decline in the expression of the genes (arrows in Fig. 4J). No expression of any of the two genes was observed in the mother pollen cells or tetrads (opened arrows pointing to reddish signals in Fig. 4N and O).
 View Details | Fig. 4. Spatial expression patterns of SlGh17, SlAPG, SlSs, SlMDL1, and SlChs in S. latifolia floral anther. Longitudinal and cross sections of anther were hybridized with digoxigeninated gene-specific antisense RNA. Detection of the transcripts is indicated by the blue/black tetrazolium blue signal. Expression of SlMDL1, SlSs, SlGh17 SlAPG, and SlChs are shown in longitudinal section of anther (A to E, respectively), cross section of anther (F to J, respectively), and inside one locule of the anther (K to O, respectively). SlMDL1, SlSs, SlGh17 showed expression in tapetum and pollen grains (filled arrows), while SlAPG, and SlChs showed expression in tapetum (filled arrows). Unfilled arrows show that there is no expression in mother pollen cells (A to E, I, N) or pollen grains (J, O). po, pollen grains; T, tetrad; t, tapetum; dt, degenerated tapetum; mp, mother pollen cells. |
SlGh17 belongs to a group of plant-encoded glycosyl hydrolase 17 family, Gh17, isozymes, which hydrolyze 1,3-β-glucan polysaccharides found in the cell wall matrix of plants and fungi. A full-length SlGh17 cDNA clone was isolated using RACE system, using the RNA isolated from the male flower buds of size 1–3.5 mm. The SlGh17 gene has an ORF of 1320 bp that encodes a product of 440 amino acids. The N-terminal region of the predicted polypeptide contains a hydrophobic domain that has characteristics of a signal peptide. Both SlGh17 and the anther-specific A6 gene (Roberts et al., 1993) of Arabidopsis thaliana contain an additional C-terminal region that is not encoded in the Gh17 genes of tobacco (Fig. 5). SlGh17 also contains two conserved regions (Meins et al., 1991) containing two Glu residues (Chen et al., 1993) and two Trp residues (Ohno et al., 1989), which appear to be important for the catalytic activity of this family (Fig. 5).
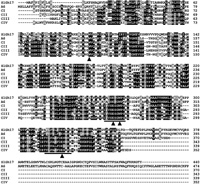 View Details | Fig. 5. Alignment of the deduced amino acid sequence of SlGh17 with those of the plant-encoded glycosyl hydrolase 17 (Gh17) family. The amino acid sequence of SlGh17 was compared to the amino acid sequences of A6 from Arabidopsis (Hird et al., 1993) and Class I (CI; GenBank accession number CAA37669 (Shinshi et al., 1988)), Class II (CII; GenBank accession number AAA34103 (Linthorst et al., 1990)), Class III (CIII; GenBank accession number CAA38324.1 (Payne et al., 1990)), and Class IV (CIV; GenBank accession number CAA38303.1 (Ori et al., 1990)) Gh17 proteins of tobacco. The regions involved in catalytic activity are underlined. The Glu and Trp residues required for catalytic activity (Chen et al., 1993) are indicated by solid arrowheads. |
A phylogenic tree based on amino acid sequences was generated to determine the evolutionary relatedness of SlGh17 to the Gh17 family proteins of other species. SlGh17 shows striking similarity to anther-specific glucosyl hydrolases (Fig. 6), and these proteins form a distinct clade on phylogenetic trees derived from sequences of various 1,3-β-glucan-hydrolyzing proteins. SlGh17 is most similar to two Brassica napus anther-specific genes expressed in the tapetum and microspores (Hird et al., 1993), the anther-specific A6 of A. thaliana (Roberts et al., 1993), and the male-flower-specific SgGN1 of the dioecious willow Salix gilgiana (Futamura et al., 2000).
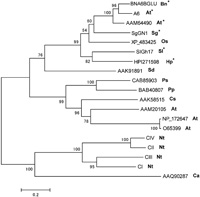 View Details | Fig. 6. Phylogenetic analysis of SlGh17. A tree generated from the alignment of the amino acid sequence of SlGh17 with those of the plant-encoded glycosyl hydrolase 17 (Gh17) family was subjected to phylogenetic analysis. The sequences in the SlGh17 clade (indicated by asterisks) are specific to anther tissues. Species are indicated after GenBank accession numbers. Ps, Pisum sativum; Bn, Brassica napus; At, Arabidopsis thaliana; Cs, Camellia sinensis; Ca, Coffea arabica; Sd, Solanum demissum; Os, Oryza sativa; Sg, Salix gilgiana; Pp, Pyrus pyrifolia; Oe, Olea europaea; Sl, Silene latifolia; Ca, Cardamine amara; Hp, Hieracium piloselloides. |
The SlChs sequence is similar to those of plant-encoded isozymes of the chalcone synthase (Chs) protein family. Chalcone synthase catalyses the synthesis of polyketides required for a large number of biologically important substances, including those required for flower color, protection from ultraviolet light, defense against pathogens, interaction with microorganisms, and fertility (Schroeder, 1997). A full-length SlChs cDNA clone was isolated using RACE system. The SlChs gene has an ORF of 1140 bp that encodes a product of 380 amino acids. The predicted polypeptide was aligned with the sequences of an anther-specific chalcone synthase of Nicotiana sylvestris, the alfa-alfa chalcone synthase, and acridone synthase (ACS; Fig. 7). SlChs contains all of the amino acid residues specific to the chalcone synthase domain (amino acids 18–380) (Jez et al., 2000). SlChs and Chs share a set of conserved catalytic residues (Cys 166, His 306, and Asn 343, marked by asterisks in Fig. 7), a similar cis-peptide turn, and CoA-binding amino acid residues (Fig. 7).
 View Details | Fig. 7. Sequence alignment of SlChs, NsChs, CHS, and ACS proteins. SlChs and NsChs are anther-specific genes, while CHS and ACS proteins are not anther specific. The catalytic amino acids residues are marked by asterisks, the cis-peptide turn residues by solid triangles, and the CoA-binding residues by dots. The locations of the amino acid residues of the active site cavity that are structural, control polyketide size, or determine substrate specificity are indicated by S, C, and U, respectively. Anther-specific residues are marked by open triangles. NsChs, anther-specific chalcone synthase of Nicotiana sylvestris; CHS, alfa-alfa chalcone synthase; ACS, Chs-like polyketide acridone synthase of Ruta graveolens. |
SlChs shares unique amino acid residues with the anther-specific chalcone synthase of Nicotiana sylvestris (open triangles in Fig. 7). Phe 56, Asp 136, Gln 161, Cys 190, Thr 197, Leu 263, Lys 269, Ala 304, and Ala 342 of CHS1 are replaced by Leu 59, Arg 138, Phe 163, Thr 192, Gly 199, Ile 264, Arg 270, Val 305, and Asn 347, respectively, in SlChs (Fig. 7). SlChs and the chalcone synthase of Nicotiana sylvestris share two protein motifs (amino acids 63–67 and 207-210 in SlChs; open triangles in Fig. 7). Changes in these conserved amino acid residues in the other proteins were identified only in anther-specific chalcone synthase genes isolated from Oryza sativa (Hihara et al., 1996), Brassica rapa (Shen and Hsu, 1992), Brassica napus (Turgut et al., 1994), tobacco (Atanassov et al., 1998), Pinus radiate (Walden et al., 1999), and Hordeum vulgare (GenBank accession number AAV49989).
A phylogenic tree based on amino acid sequences was generated to determine the evolutionary relatedness of SlChs to chalcone synthases (Chs) of other species. SlChs shows the strongest similarity with anther-specific Chs proteins, which, together with SlChs, forms a distinct clade on phylogenetic trees derived from various Chs sequences (Fig. 8). The region that encodes the chalcone synthase domain of SlChs shows high similarity to the tapetal-specific chalcone synthase gene of Oryza sativa (Hihara et al., 1996), a Brassica rapa gene expressed in the tapetum and vasculature of anthers as well as in young microspores (Shen and Hsu, 1992), a Brassica napus gene isolated on the basis of its expression in anthers (Turgut et al., 1994), a tobacco gene whose expression is restricted to the tapetum and developing microspores (Atanassov et al., 1998), and a Pinus radiata gene that is expressed only in the tapetum (Walden et al., 1999).
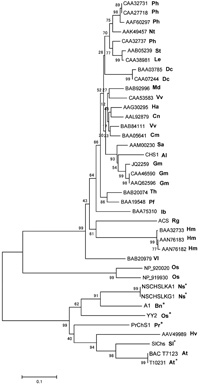 View Details | Fig. 8. Phylogenetic analysis of SlChs. A tree generated from the alignment of the amino acid sequence of SlChs with those of plant-encoded isozymes of the chalcone synthase (Chs) protein family was subjected to phylogenetic analysis. Six of the eight sequences (indicated by asterisks) in the SlChs clade are specific to anther tissues. Species are indicated after GenBank accession numbers. Ph, Petunia hybrida; Nt, Nicotiana tabacum; St, Solanum tuberosum; Le, Lycopersicon esculentum; Dc, Daucus carota; Md, Malus domestica; Ha, Hypericum androsaemum; Cn, Cannabis sativa; Vv, Vitis vinifera; Cm, Camellia sinensis; Sa, Senna alata; Gm, Glycine max; Th, Torenia hybrida; Pf, Perilla frutescens; Ib, Ipomoea batatas; Vl, Vitis labrusca; Hm, Hydrangea macrophylla; Os, Oryza sativa; At, Arabidopsis thaliana; Sl, Silene latifolia; Hv, Hordeum vulgare; Pr, Pinus radiata; Bn, Brassica napus; Ns, Nicotiana sylvestris; Al, alfa-alfa; Rg, Ruta graveolens. |
In this study, we performed a cDNA subtraction to exploit the differences in gene expression between healthy S. latifolia male buds and infected female (hermaphroditic male-sterile) buds. We isolated and characterized five cDNAs, all of which are specifically expressed in anthers. Although the smut fungus infection produces the same symptoms in both males and females, we used the infected female as the driver in the subtraction technique as it offer the isolation of male-specific cDNA that highly expressed on the later stages of anther development.
The five identified cDNAs were male-specific, showing expression in male flower buds (Fig. 1). The transcription levels of them were below the limit of detection in roots, leaves, and healthy female buds, confirming the male-specificity of the isolated clones. They were preferentially expressed in male flower buds within stages 8 to 11. In this period, the tapetum underwent a burst in RNA synthesis, beginning with meiosis and ending before the first mitotic division in the microspore (Pacini et al., 1985) (Fig. 2).
Abortion of stamen development in female flowers of S. latifolia occurs at stage 6 (Uchida et al., 2003). M. violaceum induced stamen development after this stage in infected females. Kazama et al., (2005) deteced the expression of the floral development gene, SLM2, in the induced stamens of the infected female. SLM2 was not expressed in the suppressed stamens of the healthy female, which confirms that the smut fungus can mimic the Y-chromosome domain responsible for stamen development. However, the smut fungus can not offer fertility for the induced anthers. In smut-infected female anthers little or no tapetum and also no pollen are formed. It is not clear whether the smut fungus is unable to supply a developmental cue for tapetum and pollen formation, or whether its own growth and development inside the anther loculus disrupts the formation of these tissues. In this report, we used the infected female buds, and the infected male buds to test the expression of the isolated cDNAs. The effect of fungus growth and development on the tapetum and pollen should be the same on both infected male and infected female buds. We found that the isolated cDNAs were expressed in the infected male buds, which has a signal for the development of the tapetum and pollen grains from the Y chromosome (Fig. 1). In contrast, the isolated cDNA showed no expression in the infected female (Fig. 1). We could propose that the smut fungus is unable to supply a developmental signal for tapetum and pollen grains, and we could eliminate the possibility that its growth and development destroy these tissues. Also, we could propose that these genes are linked to the master control gene(s) on the Y chromosome.
In situ hybridization patterns show that the isolated genes can be classified into two groups. SlMDL1, SlSs and, SlGh17 were expressed in both tapetum and pollen grains (Fig. 4). The main physiological role of the MDL protein is to catalyze the dissociation of (R)-mandelonitrile to HCN and an aldehyde or ketone (Yemm and Poulton, 1986). As hydrogen cyanide is toxic to most aerobic organisms, and its large-scale production occurs only upon tissue damage, it has been suggested that cyanogenic compounds, in concert with their catabolizing enzymes, aid in defending these plants against pathogens, thus avoiding damage to the plant tissues (Swain et al., 1992). Another hypothesis, which does not exclude the previous one, is that these cyanogenic glycosides and lipids serve as storage compounds for reduced nitrogen, since they are used for the synthesis of non-cyanogenic compounds during seedling development in Hevea brasiliensis and Ungnadia speciosa (Selmar et al., 1988). Strictosidine synthase (Str) catalyzes the condensation of tryptamine and secologanin to form strictosidine, the universal precursor of terpenoid indole alkaloids in plants. Suelves and Puigdomenech, (1998) reported that MDL accumulates in stamens and flower buds of Prunus amygdalus. Strong expression of Str genes was reported in sugarcane flower buds, but the expression of these genes has also been reported in other plant tissues such as roots and leaves (França et al., 2001), and no precise functions in flowers have been reported for these genes. To our knowledge, this is the first report of anther-specific MDL and Str genes.
SlGh17 belongs to a family of plant-encoded isozymes of the Gh17 family. Gh17 enzymes are associated with plant defenses against pathogen attack and stress (Van Loon and Van Kammen, 1970), but are also expressed in floral tissues (Lotan et al., 1989). The anther-specific genes of B. napus and the dioecious willow Salix gilgiana also contain this C-terminal domain (not shown in Fig. 5), which appears to be important for the functions of the proteins in this Gh17 sub-tree. In a phylogenetic tree, the isolated SlGh17 groups with a small subset of anther-specific proteins (Fig. 6). Our in situ hybridization results confirm the strong expression of SlGh17 in tetrads and pollen grains (Fig. 4). In accordance with our results, Hird et al., (1993) showed expression of the anther-specific Gh17 gene in Arabidopsis tapetum and pollen grain, with maximal expression observed just prior to tetrad dissolution. Futamura et al., (2000) reported the absence of tapetum-specific expression and the accumulation of Gh17 in tetrads and pollen grains of the dioecious willow Salix gilgiana. The SlGh17 might play an important role in the development of mature pollen grain, by degrading the callose wall of the tetrad of the microspores in S. latifolia.
SlAPG and SlChs were only expressed in the tapetum (Fig. 4). SlAPG shows strong similarity to anther-specific proline-rich protein APG precursors of A. thaliana and O. sativa. Genomic and cDNA clones of the anther-specific APG genes of A. thaliana and B. napus have been characterized (Roberts et al., 1993). In accordance with our genomic Southern results for SlAPG (Fig. 3), Southern blotting and Northern analysis of male-fertile and cytoplasmic-male-sterile varieties of B. napus showed that the APG gene is present as a single copy in the Arabidopsis genome and that the B. napus APG gene is a member of a small anther-specific gene family. Reporter gene fusions established that the APG promoter directs expression in a number of cell types in anthers of transformed plants (Roberts et al., 1993).
SlChs belongs to a group of plant-encoded isozymes of the chalcone synthase family. Chalcone synthases catalyze the synthesis of the polyketides required for a number of biologically important functions, such as flower color, protection from ultraviolet light, defense against pathogens, interaction with microorganisms, and fertility (Schroeder, 1997). We identified anther-specific amino acid residues in SlChs and showed that these residues are conserved in all anther-specific proteins of the Chs family (Fig. 7). These variations in the amino acid residues likely lead to different substrate preferences and different polyketide products (Jez et al., 2000), which could explain the functions of the Chs proteins in the male reproductive tissues.
Because the SlChs clade (Fig. 8) contains members from S. latifolia, pine, rice, tobacco, and Brassica spp., the evolution of this clade must have occurred before the divergence of gymnosperms and angiosperms. In contrast, stilbene synthase genes evolved from Chs genes on several occasions during the course of evolution within both the angiosperm and gymnosperm divisions of the plant kingdom (Tropf et al., 1994). Chs enzymes have been demonstrated to be essential for pollen development. Disrupting the Chs activity in the anthers of petunia (Van der Meer et al., 1990), maize (Coe et al., 1981), and tobacco (Fischer et al., 1997) results in the production of sterile pollen that can be rescued by the application of exogenous flavonoids (Fischer et al., 1997).
In summary, we have shown that homologous genes are expressed differentially during the male reproductive development of the dioecious plant S. latifolia. These genes should be related to the fertility of the anther, leading to the development of fertile pollen, and under the control of master gene(s) in the Y chromosom. Due to the spatial and stage-specific nature of their expression, these genes will be useful for the isolation of anther-specific promoters, understanding the genetic manipulation of male sterility, and revealing the interactions between the male-sterility restoration factor and fertility-related genes.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (to S. K., 15013215) and a Grant-in-Aid for the 21st Century COE Program to Tokyo University (to A. A.) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
|