| Edited by Kazuhiro Kutsukake. Hiroshi Hara: Corresponding author. E-mail: hhara@post.saitama-u.ac.jp |
Enteric bacteria including Escherichia coli synthesize an extracellular polysaccharide capsule, called colanic acid. The Rcs signal transduction system positively regulates the expression of the capsular polysaccharide synthesis (cps) operon (Stout, 1994; Majdalani and Gottesman, 2005). It is a multistep phosphorelay system, in which RcsC sensor kinase is autophosphorylated in response to environmental stimuli and activates RcsB response regulator by phosphoryl group transfer via YojN (also called RcsD), a phosphotransmitter protein containing an HPt-domain (Takeda et al., 2001). The phosphorylated RcsB acts as a DNA-binding transcriptional regulator and activates the cps transcription by forming a heterodimer with RcsA, which is not a component of the phosphorelay but acts as a coregulator. Many genes in addition to the cps operon have been shown to be regulated by the Rcs system. Some are dependent on RcsA as is the cps operon, and others are regulated by RcsB homodimer independently of RcsA (Majdalani and Gottesman, 2005).
The RcsF protein was first reported to activate the Rcs system when overproduced (Gervais and Drapeau, 1992) and was thought to be an accessory positive factor of the system. It was subsequently shown to be essential for the Rcs activation in response to glucose and zinc ion at 20°C (Hagiwara et al., 2003), lack of major acidic phospholipids, lack of membrane-derived oligosaccharides (MDOs), treatment with a cationic amphipathic compound chlorpromazine (Shiba et al., 2004), and a defect in lipopolysaccharide biosynthesis (Majdalani et al., 2005; our unpublished result), indicating that RcsF is an inherent component of this signal transduction system. This protein was predicted to be an outer membrane lipoprotein based on its amino acid sequence (Hagiwara et al., 2003; Shiba et al., 2004; Majdalani et al., 2005), and a sucrose density gradient centrifugation experiment confirmed its localization to the outer membrane (our unpublished data).
It was reported that overexpression of djlA , lolA, and ompG genes activated the Rcs system and elevated transcription of the cps operon (Clarke et al., 1997; Kelley and Georgopoulos, 1997; Chen et al., 2001). DjlA, a type III cytoplasmic membrane protein with a very short N-terminal periplasmic segment preceding a transmembrane segment and a large C-terminal cytoplasmic region, is a member of the DnaJ family that includes DnaJ and CbpA of E. coli (Clarke et al., 1996). DjlA overproduction was used to stimulate the Rcs signaling in the analyses of this signal transduction system (Takeda et al., 2001; Ferrières and Clarke, 2003). LolA is a periplasmic soluble protein and acts as an outer membrane lipoprotein-specific transporter (Matsuyama et al., 1995). Its function is essential for cell viability even in the absence of major outer membrane lipoprotein Lpp (Tajima et al., 1998). OmpG is an outer membrane porin with an unusually large channel size (Fajardo et al., 1998).
RcsF, first identified as a multicopy activator of the Rcs system, turned out to be a component of the system. Likewise, DjlA, LolA, and OmpG may be essential components or accessory positive factors of the Rcs system. Otherwise, excess amounts of these cytoplasmic membrane, periplasmic, and outer membrane proteins may produce envelope stresses that stimulate the Rcs signaling. In this study we examined the possible involvement of DjlA and OmpG in regulation of the Rcs system.
Deletion of the genes for essential components or accessory positive factors of the Rcs system would abolish or decrease the activation of the cps transcription by the system. LolA could not be subjected to such examination, however, because disruption of the lolA gene is lethal (Tajima et al., 1998). We transduced ΔompG::kan and ΔdjlA::kan alleles by phage P1 into cpsB’-lac fusion strains CL332 (pgsA-null) and CL034 (mdoH-null) lacking major acidic phospholipids and MDOs, respectively, and thereby showing stimulated Rcs signaling (Ebel et al., 1997; Shiba et al., 2004; see Table 1 for the E. coli K-12 derivative strains and plasmids used in this study). Cells were grown in buffered Luria-Bertani (LB) medium (Shiba et al., 2004) at 30°C to mid-exponential phase (ca. 100 units of Klett-Summerson colorimeter equipped with a no. 54 filter) and assayed for β-galactosidase activity using o-nitrophenyl-β-D-galactoside (ONPG) as a substrate at 28°C. The assay method and the unit definition were as described by Wang and Doi (1984).
 View Details | Table 1. Bacterial strains and plasmids used in this study |
As shown in Fig. 1, the ompG deletion had no influence on Rcs activation level, indicating that OmpG is neither an essential component nor a positive regulator of the Rcs system. The ompG gene is not expressed in wild-type E. coli K-12 strains due to the absence of an active promoter. Its expression was observed only when a large chromosomal deletion placed it under the control of the pspA promoter normally located far upstream (Fajardo et al., 1998). Such a gene is not supposed to play an important positive role in regulation of signal transduction. Artifactual overproduction of this outer membrane porin forming an unusually large channel (Fajardo et al., 1998) probably brings on a stressful condition that activates the Rcs system.
 View Details | Fig. 1. Effects of the ompG and djlA deletion mutations on the Rcs signal transduction system in strains lacking major acidic phospholipids (pgsA-null) and MDOs (mdoH-null). The mutations transduced into CL332 and CL034 were ΔompG::kan and ΔdjlA::kan. The activity of the Rcs system was monitored by the activity of β-galactosidase directed by the cpsB’-lac transcriptional fusion. The means and standard deviations of three measurements are shown. |
On the other hand, the djlA deletion unexpectedly resulted in increased activation of the Rcs system compared with the parent strains, although the increase levels were different between the pgsA and mdoH mutants (Fig. 1). Thus DjlA is neither an essential component nor a positive regulator of the Rcs system. The increased activity rather indicated that DjlA might act as a negative regulator at a physiological expression level. Alternatively, the djlA deletion might lead to production of a stimulus additive to that due to lack of major acidic phospholipids or MDOs, causing an additive Rcs-activating effect. Although we cannot completely exclude a possible effect of the djlA deletion on cellular levels of the Rcs proteins, it was reported that DjlA did not affect the expression of the rcs genes (Kelley and Georgopoulos, 1997). Since the djlA gene is followed by a ρ-independent transcription terminator and the downstream yabP gene is transcribed separately from djlA (Bernard et al., 1998), it is unlikely that transcription of the kanamycin resistance gene (kan) inserted in the ΔdjlA::kan allele affects the expression of the downstream genes.
If DjlA acts as a negative regulator, an increase in its cellular level should decrease the cps transcription. We transformed an mdoH cpsB’-lac strain CL034 with pHR741, a low-copy-number plasmid carrying djlA under a weakened derivative of Ptrc promoter (Shiba et al., 2004). In the β-galactosidase assays in the absence of isopropyl-β-D-thiogalactoside (IPTG), the cps transcription level of the transformants was about three fourths of that exhibited by CL034 transformed with the vector plasmid, pHR719 (Fig. 2). Even in the absence of the inducer, djlA should be expressed from the plasmid-borne copy at a basal level of the inducible promoter, in addition to the chromosomal copy. Such a low-level overproduction of DjlA repressed the Rcs activation due to lack of MDOs. This indicates that DjlA acts as a negative regulator of the Rcs system. The effect of the djlA deletion is due more likely to loss of the negative regulation than to production of an Rcs-activating stimulus.
 View Details | Fig. 2. Effects of low- and high-level overexpression of djlA on the Rcs system in the mdoH-null strain. CL034 cells harboring a low-copy-number plasmid pHR741 carrying djlA under an IPTG-regulatable promoter and the vector plasmid pHR719 were grown in the absence (the left vertical axis) or presence (the right axis) of 100 μM IPTG at 30°C and assayed for the activity of β-galactosidase directed by the cpsB’-lac transcriptional fusion. The means and standard deviations of three measurements are shown. |
Strong induction of djlA with 100 μM IPTG greatly increased the cps transcription, consistent with the previous reports (Clarke et al., 1997; Kelley and Georgopoulos, 1997). Thus DjlA can affect the Rcs system either negatively or positively depending on its expression level. A condition that strongly induces expression of the chromosomal djlA gene is not known. At a physiological expression level DjlA negatively regulates the system.
To negatively regulate the Rcs system stimulated in the pgsA- or mdoH-null mutant, DjlA might relieve the stresses posed on the envelope by lack of major acidic phospholipids or MDOs or directly repress the activity of the system. We examined the effect of the djlA deletion in a strain wild-type for phospholipids and MDOs. The ΔdjlA::kan allele was transduced into UE29, the parent strain of CL332 and CL034. In β-galactosidase assays using ONPG as a substrate the transductants showed a higher level of cps transcription than the parent, although the β-galactosidase activity in the pgsA+ mdoH+ background and its increase in the ΔdjlA transductants were very small (Fig. 3A). Experiments on LB agar medium containing 40 μg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-Gal) gave essentially the same results: colonies of the ΔdjlA transductants developed blue color, very pale but clearly distinguishable from white colonies of UE29, at 30°C (Fig. 3B). Thus the effect of the djlA deletion was observed in the absence of a stress due to the lack of major acidic phospholipids or MDOs.
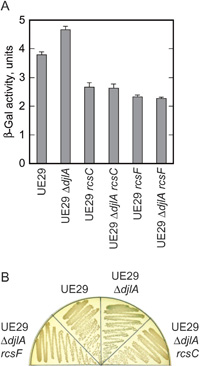 View Details | Fig. 3. Effects of the djlA deletion mutation on the Rcs system in a strain wild-type for MDOs and phospholipids. The rcs mutations transduced into UE29 and UE29 ΔdjlA::kan strains were rcsC::mini-Tn10 cam and rcsF::mini-Tn10 cam. A, Cells were grown to ca. 100 Klett units at 30°C and assayed for the activity of β-galactosidase directed by the cpsB’-lac transcriptional fusion. The means and standard deviations of three measurements are shown. B, Overnight cultures were diluted to 1 Klett unit, streaked on LB agar medium containing X-Gal, and incubated at 30°C overnight. |
If lack of DjlA protein were a cause of an Rcs-stimulating stress by itself, the djlA deletion should have a much larger additive effect of elevating the β-galactosidase activity by ten units or more as found in the pgsA- or mdoH-null background (Fig. 1). However, the increase in the cpsB’-lac transcription due to the djlA deletion was small. This is consistent with our postulation that the effect of ΔdjlA is not due to production of an envelope stress.
When the rcsC or rcsF gene was disrupted by mini-Tn10 cam (Shiba et al., 2004), UE29 showed lower cps transcription (Fig. 3A). A similar result was reported by Majdalani et al. (2005). These results indicate that RcsB is phosphorylated at a low level in an RcsC- and RcsF-dependent manner even in the apparent absence of a stimulus so far known to activate the Rcs system. When such a besal-level phosphorelay of the Rcs system was lost in rcsC- or rcsF-defective mutants, the cpsB’-lac fusion showed even lower transcription, which is attributable to cross-talk phosphorylation of RcsB as suggested by Majdalani et al. (2005). This cross-talk phosphorylation of RcsB independent of the Rcs phosphorelay was not affected by ΔdjlA::kan mutation (Fig. 3A and B). Thus loss of DjlA led to derepression of an RcsC- and RcsF-dependent basal-level phosphorelay of the Rcs system. Probably DjlA negatively regulates the function of RcsC and/or YojN by interacting with these cytoplasmic membrane proteins as we will discuss below.
The result with the rcsF-disrupted ΔdjlA strain (Fig. 3) also supports our notion that the djlA deletion does not lead to production of an Rcs-stimulating stress. If there were such a stress, no increase of the cps transcription in the absence of RcsF should indicate that the stress stimulated the Rcs system via RcsF as other envelope stresses do. However, it seems unlikely that lack of DjlA, a cytoplasmic membrane protein whose most part is located in the cytoplasm, caused a stress affecting cytoplasmic membrane-located RcsC sensor kinase via outer membrane-located RcsF.
We conclude that lack of DjlA is not a cause of an Rcs-stimulating stress and that DjlA acts as a negative regulator of the Rcs phosphorelay system not only when the system is highly activated but also when it is only weakly operating at its basal level.
Transcriptome analyses identified a large number of genes under direct or indirect control of the Rcs system (Oshima et al., 2002; Ferrières and Clarke, 2003; Hagiwara et al., 2003). Their control is important for bacterial cells to be adapted to various environmental changes encountered outside of a mammalian host (Stout, 1994) and during biofilm formation (Ferrières and Clarke, 2003). The negative regulation by DjlA presumably functions to keep a low basal-level activity of the Rcs system, to prevent its overactivation when responding to environmental stimuli, and to facilitate recovery to the basal level when the stimuli disappear. There is another cytoplasmic membrane protein YrfF likely to regulate the system negatively: its homolog in Salmonella enterica, IgaA, was reported to repress the Rcs activity (Cano et al., 2002). The repression by Salmonella IgaA is crucial for the virulence of this intracellular pathogen (Domínguez-Bernal et al., 2004). In Legionella dumoffii DjlA is required for intracellular growth (Ohnishi et al., 2004), which may be because of its negative regulatory function on the Rcs system.
Considering the membrane topology of DjlA, which has only six N-terminal residues on the periplasmic side of a single transmembrane segment (Clarke et al., 1996), its large C-terminal cytoplasmic region possibly functions to negatively regulate the Rcs system by interacting RcsC and/or YojN, which have histidine kinase and phosphotransmitter domains, respectively, in their large cytoplasmic regions, and by affecting some step of the phosphorelay process starting with autophosphorylation of RcsC. It is also possible that the transmembrane segment of DjlA interacts with the transmembrane segments of RcsC and/or YojN and affects the transmembrane signal transmission. RcsC and YojN have two transmembrane segments sandwiching a periplasmic domain in their N-terminal regions. It has been postulated that a movement in the bundle of transmembrane α-helices of multimeric sensor proteins transmits a signal sensed by the extracytoplasmic domain to the cytoplasmic histidine kinase domain (Chervitz and Falke, 1996).
Activation of the Rcs system by overproduced DjlA requires the J-domain at the C terminus (Kelley and Georgopoulos, 1997; Genevaux et al., 2001) and the transmembrane segment (Clarke et al., 1997) and was suggested to involve specific interaction of these regions with RcsC (YojN was not known as a component of the Rcs system when such a suggestion was made). The activation is independent of RcsF (Shiba et al., 2004), indicating that DjlA affects the Rcs system by a different mechanism from other envelope stresses known to cause an RcsF-dependent activation of the system. Although the DjlA overproduction is an artifactual situation and has an effect opposite to that of DjlA of a physiological expression level, we propose that the negative regulation by DjlA demonstrated in this study also involves specific interaction of the transmembrane and C-terminal regions of DjlA with the corresponding regions of RcsC and/or YojN.
J-domain is a sequence conserved among DnaJ family proteins and essential for their interaction with DnaK chaperone and activation of its ATPase activity. DjlA can substitute for DnaJ co-chaperone (Genevaux et al., 2001) and seems to have multiple functions in addition to the Rcs regulation. Although the co-chaperone activity of DjlA might affect the activity of intrinsically unstable RcsA protein, it would rather positively regulate the cps transcription: DnaJ was reported to be necessary for keeping RcsA soluble and active (Jubete et al., 1996). Interaction of DjlA with DnaK (Genevaux et al., 2001) suggests another intriguing possibility that the Rcs system may respond not only to environmental stimuli but also to intercellular conditions through indirect interaction with DnaK mediated by DjlA.
We are grateful to Hirotada Mori, Tomoya Baba, and Barry L. Wanner for strains of KO collection. This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.