| Edited by Fujio Kawamura. Kouji Matsumoto: Corresponding author. E-mail: koumatsu@molbiol.saitama-u.ac.jp |
The recent development of probes that exclusively recognize phospholipids with particular polar head groups has made it possible to localize specific phospholipids in bacterial cells and to show that phospholipids are distributed heterogeneously, not homogeneously, in bacterial membranes (Dowhan et al., 2004; Kawai et al., 2004, Mileykovskaya and Dowhan, 2000; Nishibori et al., 2005). Cardiolipin (CL), an anionic phospholipid, has been shown to be localized at the septal and on the polar membranes in Escherichia coli cells with the CL-specific fluorescent dye 10-N-nonylacridine orange (NAO) (Mileykovskaya and Dowhan, 2000; Mileykovskaya et al., 2001). It has been suggested that CL plays specific roles in essential cellular processes including the initiation of DNA replication and the processes of cell division (Mileykovskaya and Dowhan, 2005). Nevertheless, the significance of CL in vivo is still cast in doubt by its dispensability in both E. coli and B. subtilis (Kawai et al., 2004; Kikuchi et al., 2000; Matsumoto, 2001). B. subtilis has three structural genes (clsA, ywjE and ywiE) for CL synthase. The dominant role in CL synthesis is played by clsA; however, disruption of clsA, which causes the level of CL in exponential growth phase cells to drop below the detectable level, gives no phenotypic change (Kawai et al., 2004). The contribution of ywjE and ywiE to CL synthesis has not been clarified yet.
We have suggested that CL rich domains are localized at the septal and on the polar membranes in B. subtilis cells based on experiments using NAO (Kawai et al., 2004). In that study we also found increased NAO-fluorescence in polar septal, engulfment and on forespore membranes during the sporulation phase and have shown that CL content is indeed higher in sporulation phase cells, an apparent similarity to E. coli, which has an increased CL content in the stationary phase (Nishijima et al., 1988). One likely reason for the high CL content in sporulating cell membranes is that it is needed in the sporulation process. The clsA-disrupted mutants, which lack a detectable level of CL in the exponential growth phase but have a trace amount of CL in the cells in the sporulation phase, show a delay in the process of sporulation and form fewer heat-resistant spores (Kawai et al., 2004). Another reason may be that CL is needed in spore membranes, because it probably plays a vital role in the dormant and/or the germinating state. Spore membranes would thus be expected to have high CL content. However, there is no data on the lipid composition of spore membranes, since the extraction of membrane lipids from B. subtilis spores is hampered by thick rigid coat layers and a cortex that protect the spore membranes and protoplast from attack by a wide variety of toxic molecules, heat, UV irradiation and mechanical disruption (Driks, 2002; Moir, 1981; Moir and Smith, 1990).
In order to extract lipids from the spore membranes, we adopted methods that dissolve the rigid coat. We also examined cotE and gerE mutant spores, which have a defective, lysozyme permeable coat. Analysis of the extracted lipids revealed that the lipid of spore membranes contains a higher percentage of CL than the membrane lipid of exponentially growing cells. To explore the role that CL plays in spore membranes we then examined the possibility of the involvement of CL in germination. Spores germinate upon addition of nutrients such as L-alanine or AGFK, which are recognized by specific germinant receptors. The turbidity of spore suspensions decreases because of the darkening of refractive spores after release of dipicolinic acid (DPA) and rehydration of the core protoplast (Moir and Smith, 1990). We examined the germination of the spores of CL synthase mutants containing only a trace amount of CL and found that no turbidity decrease was induced by addition of the germinants.
The B. subtilis Marburg strains used in this study are listed in Table 1. Wild type strain 168 of B. subtilis Marburg and the strains BFS219 (clsA::pMutin4), which is defective in clsA, and SDB206 (clsA::pMutin4 ywiE2::neo ywjE1::spc), which is defective in all three paralogous genes for CL synthase, have been described previously (Kawai et al., 2004). SDB210 (clsA::pMutin4 ywiE2::neo ywjE1::spc ΔpssA::cat) was constructed by transformation of the SDB206 strain with the DNA of SDB211, which has a null ΔpssA::cat allele. For construction of the ΔpssA::cat allele a 1,399 bp segment of the upstream region from the initiation codon of pssA was PCR amplified with the primer pair PssAatII (5’-tgaggagccgacgtcagagattggcg-3’) and PssasXbaI (5’-cgatagtgatcgtctagagttacagg-3’) and a 1,129 bp segment of the downstream region from the 127 bp upstream of the stop codon was PCR amplified with the primer pair PssXbaI (5’-cttcacctctagaccgatccttctggc-3’) and PssasBamHI (5’-cgtatttggatcctagttgatttaccggg-3’). Both the upstream and downstream regions of pssA were inserted into AatII/BamHI digested pBR322. The constructed plasmid was digested with XbaI and then the cat block of a 1 kbp segment that was PCR amplified with a primer pair catXbaI (5’-cttctagagaaactcaacgagctggacgcgg-3’) and catasXbaI (5’-acctctagaactaacggggc-3’) using a template DNA of pDHCMGFP, cited in Nishibori et al. (2005), was inserted to yield a plasmid pFKPSS211 that harbors the null allele ΔpssA::cat. This plasmid was introduced into B. subtilis 168 by double crossover recombination.
 View Details | Table 1. Bacillus subtilis Marburg strains |
The mutant strains TF35 and COTE5C, both defective in spore coat formation, were also used. The TF35 strain that harbors gerE::cat has been described in Kuwana et al. (2003). The gerE disruption causes a defect in spore coat formation and makes the spore sensitive to lysozyme as described by Moir (1981). For construction of the cotE mutant COTE5C, a 156 bp segment, from the 19 bp downstream from the initiation codon of cotE, the structural gene for an alkaline-soluble coat protein of 24 kDa (Zheng et al., 1988), was PCR amplified with primers COTE19 (5’-aggaagcttattacgaaggcagtagtag-3’) and COTE174R (5’-catggatcccttcaatttctaccgttt-3’), cut with HindIII and BamHI at primer-based sites, and inserted into a HindIII/BamHI-digested pCAT5 vector (Kuwana et al., 2002) to obtain the plasmid pCOTE5C. This plasmid was introduced into B. subtilis 168 by a single crossover recombination and selected by chloramphenicol resistance (5 μg/ml chloramphenicol) to yield strain COTE5C.
The wild type and mutant strains were cultivated in sporulation medium (DSM), which contained 0.8% nutrient broth (Difco), 0.1% KCl, 0.025% MgSO4·7H2O, 1.0 mM Ca(NO3)2, 10 μM MnCl2, and 1.0 μM FeSO4 (Schaeffer et al., 1965). Luria-Bertani (LB) broth and Tris-HCl (pH 8.0) buffer were used for germination experiments. L-alanine (10 mM) or the combination of 10 mM each of asparagine, glucose, fructose, and KCl (AGFK) were added as germinants in Tris-HCl buffer. When required, the following supplements were added to the media (per liter); 20 mg of neomycin (Wako Pure Chem.), 50 mg of spectinomycin (Sigma), 0.3 mg of erythromycin (Sigma), or 5 mg of chloramphenicol (Sigma). For [14C]-labeling of membrane lipids 0.5 μ Ci per ml of [1-14C] acetic acid (57.2 mCi/mmol, Amersham) was included and cells were harvested during the late exponential phase or after three days of cultivation at 37°C. Spores of the wild type and the mutant strains were purified from three-day DSM cultures by the PEG two-phase method of Sacks and Alderton (1961). After repeated washing with distilled water the purity of the preparation was high; it contained over 99% refractive spores when assessed with a phase contrast microscope. Germination of the mutant spores was examined as follows. Purified spore preparations were heat activated (75°C) for 30 min, inoculated into LB medium or Tris-HCl buffer containing 10 mM L-alanine or AGFK, and the turbidity decrease of the spore suspension was measured with a Klett-Summerson colorimeter (no. 54 filter).
Spore breakage was accomplished by two procedures. i) Sensitization of spores to lysozyme was performed by a modification of the method described by Buchanan and Neyman (1986). The coat proteins of spores were dissolved by treatment with 50 mM dithiothreitol-8 M urea for 1h at 0°C, washed twice with distilled water, then treated with 0.1 M NaOH for 15 min at 0°C. After the final wash with distilled water, the stripped spores were digested with 20 mg/ml lysozyme for 10 min at 0°C, after which they were collected by centrifugation and subjected to lipid extraction. ii) Spores of cotE and gerE mutants were directly digested with lysozyme (20 mg/ml at 0°C) for 10 min to extract lipids, since the coat protein-defective mutant spores are sensitive to lysozyme (Moir, 1981; Zheng et al., 1988). The digested samples were collected and subjected to lipid extraction according to the method described previously (Matsumoto et al., 1998). These procedures probably extract all lipid from both inner and outer spore membranes (cf. Hudson et al. 2001), although in the first procedure a portion of outer membranes may be lost during the repeated washing by centrifugation.
In order to examine the lipid composition of spore membranes, the rigid spore coat was broken down and stripped off spore particles. Alternatively, mutant spores with defective coat were used. Both the chemically stripped and the mutant spores are sensitive to lysozyme and organic solvent (Buchanan and Neyman, 1986; Moir, 1981; Zheng et al., 1988). The lipids extracted after chemical removal (dithiothreitol/urea and alkaline treatment) of the rigid coat followed by lysozyme treatment of the spores of the wild type strain 168 were extremely rich in CL. The CL content amounted to 26.5% of total phospholipids in contrast to the value of 2.3% for exponential growth phase cells extracted under the same conditions (Table 2).
 View Details | Table 2. Phospholipid composition of spore membranes of wild type B. subtilis |
Lysozyme digestion of the mutant spores with defective coat was carried out to extract lipids from spore membranes. We could have avoided lysozyme treatment, but it proved indispensable to obtain effective separation of the lipids on subsequent thin layer chromatography. Without lysozyme treatment certain slowly migrating materials, not detectable with Dittmer-Lester reagent, appeared to hamper the migration of the phospholipids of low Rf values. The apparent CL content, according to this assessment, of the cotE::cat mutant and gerE::cat mutant spores was ca. 32% and 31% of total phospholipids, respectively. We note that even this mild treatment for lipid extraction indicated a high CL content in spore membranes.
In order to further confirm the CL accumulation in spore membranes, we carried out [14C]-labeling of spore membrane lipid. The cells of the wild type and the coat-defective mutant strains were cultivated in DSM containing [1-14C]-labeled acetic acid for three days at 37°C. Spores were collected and the membrane lipids of wild type spores were extracted after chemical removal of the rigid coat followed by lysozyme treatment and coat-defective mutant spores were extracted after direct lysozyme treatment. Two-dimensional thin-layer chromatography of these lipid preparations indicated a high CL content in the spore membranes. Typical autoradiograms of the wild type lipid preparations are shown in Fig. 1. Wild type, cotE mutant, and gerE mutant spore membranes had a CL content of 24.3%, 23.9% and 26.0% of total lipids, respectively (Table 3). The CL increase was accompanied by a decrease (ca.1/3 of that of the exponentially growing cells) in phosphatidylethanolamine content and a decrease in the content of lysylphosphatidylglycerol. Diglucosyldiacylglycerol content was also decreased in the spore membranes. However, no significant change in phosphatidylglycerol content was observed. As both lysylphosphatidylglycerol and phosphatidylethanolamine are zwitterionic, their decrease amplifies the increase in anionic nature, which is caused by the CL increase, to make the polar surface of the membranes highly anionic. We suggest that the highly anionic nature of the spore membranes plays a vital role in dormant spores and/or in germinating spores (e.g. in tethering and/or recruiting certain basic molecules to the spore membranes).
 View Details | Fig. 1. Autoradiograms demonstrating CL enrichment in B. subtilis spore membranes. Cells of B. subtilis Marburg wild type were cultivated at 37°C in DSM medium containing 0.5 μ Ci/ml of [1-14C]acetic acid and harvested in the late exponential growth phase (a) and after 3 days of incubation (b). Both spores and the exponential phase cells were treated with 50 mM dithiothreitol-8 M urea for 1h at 0°C and then cold 0.1 M NaOH for 15 min, and the treated samples were digested with lysozyme (20 mg/ml) at 0°C for 10 min. The digested samples were subjected to lipid extraction. The lipid fractions were then subjected to two-dimensional TLC. PE, phosphatidylethanolamine; PG, phosphatidylglycerol; CL, cardiolipin; lysylPG, lysylphosphatidylglycerol; MGDG, DGDG, TGDG; mono-, di-, triglucosyldiacylglycerol, respectively. |
 View Details | Table 3. Lipid composition of spore membranes of wild type, clsA, and coat-defective mutant strains of B. subtilis |
The spore membranes of the SDB206 strain with disruptions in all three paralogous genes for CL synthase had a CL content of about 0.9% of the total lipids (Table 3). This contrasts with the absence of a detectable level of CL in the cell membranes of logarithmic phase cells of the mutants (Kawai et al., 2004). It is, however, consistent with the presence of a small amount of CL (0.3% of the total lipids) during the sporulation phase (at T4) (Kawai et al., 2004). What process is responsible for the production of CL in the membranes of the cells during sporulation and in spores? Phosphatidylserine synthase of E. coli, the product of pssA, produces CL in the clsA null mutant of E. coli; the amount of CL produced depends on the gene dosage of pssA (Nishijima et al., 1988). This is also the case when the B. subtilis pssA gene is introduced into the clsA null mutant of E. coli (Saha, 1996). We therefore introduced a disrupted pssA allele, ΔpssA::cat, into the triply disrupted mutant SDB206 to construct a mutant with quadruple disruption, SDB210, in order to examine the effect of pssA on CL content in B. subtilis cells. The CL content of the quadruple mutant was decreased by a small amount (0.4%) from that (0.9% of the total) of the triple mutant SDB206 (Table 3), suggesting a possible involvement of pssA in CL synthesis in B. subtilis cells. A very small but significant amount, 0.5%, of CL was, however, still observed in the quadruple mutant SDB210. NAO staining of the sporulation phase cells of the quadruple mutant at stage T4 produced fluorescence on the engulfment membranes (data not shown), indicating the presence of CL in spore membranes. These results suggest that B. subtilis has a mechanism for accumulating CL in forespore and spore membranes, which may include a yet unknown route of CL synthesis.
The increased CL content during the sporulation phase (Kawai et al., 2004) and in spores (this study) can perhaps be understood by considering the results of β-galactosidase transcriptional fusion experiments indicating that both clsA and ywjE exhibit their maximal activities during the early sporulating phase but that ywjE has very low activity (Kawai et al., 2004). Transcriptional profiling showing that the mRNA level of clsA and ywjE exhibits a σE-dependent increase by 2.1 and 11.7, respectively (Eichenberger et al., 2003), provides supporting evidence. Recent temporal profiling (Steil et al., 2005) also indicates a σG-dependent increase of ywjE. The hypothesis that the gene product of pssA is involved in CL production in spore membranes is consistent with the observation that pssA is apparently activated upon entry into the sporulation phase by σX and σW (Cao and Helmann, 2004). Another conceivable route of CL synthesis, which is suggested by the presence of CL in the quadruple mutant spore, might involve a side reaction of certain lipid synthesis enzymes, the expression of whose genes may depend on sporulation specific sigma factors. If B. subtilis cells have an ATP-dependent CL specific lipase, like the CL-specific phosphodiesterase in gram-negative bacteria (Cole and Proulx, 1975), it might play a role in the accumulation of CL in the membranes of the cells in sporulation.
Taken together, it is evident that B. subtilis spore membranes are enriched with CL, though the provenance of the small amount of CL in the spore membranes of the CL synthase mutant is not yet fully explained. What, then, is the function of the large amount of CL in spore membranes?
Upon addition of nutrients the turbidity of wild type spore suspension decreases to 50% within 30 min due to darkening of refractive spores after release of Ca ++ and dipicolinic acid (DPA), and rehydration of the core protoplast (Moir and Smith, 1990). The subsequent outgrowth increases turbidity again. Nutrients such as L-alanine and AGFK are known germinants recognized by specific germinant receptors, GerA and GerB/K, respectively (Moir and Smith, 1990; Paidhungat and Setlow, 2002).
The turbidity decrease observed after inoculation of wild type spores into LB liquid medium was found to be much delayed and very small for SDB206 spores with a trace of CL and the following outgrowth was greatly reduced (Fig. 2). This delayed turbidity decrease was also observed with the spores of clsA-disruptant BFS219, which has only a trace amount of CL in spore membranes (as does SDB206), but not with the spores of ywjE-disruptant SDB201, which has normal CL content (data not shown). The turbidity decrease was normal with the spores of ΔpssA-mutant SDB211, which lacks phosphatidylethanolamine but has a normal level of CL. We anticipated that an initial step of germination, probably a function that involves germinant receptors, was impaired under the abnormal conditions of the mutant membranes with only a trace of CL. In order to clarify whether the impairment in the turbidity decrease in the mutant spores with trace CL involves germinant receptors and which receptor is responsible for triggering the CL-dependent turbidity decrease, we examined the effect of the known germinants L-alanine and AGFK separately. Addition of L-alanine (10 mM) in Tris-HCl buffer did not induce a turbidity decrease in the case of the mutants with trace CL, although turbidity of the wild type and ΔpssA-mutant spores decreased to 50% within 30 min (Fig. 3A). At 60 min SDB206 showed a decrease of 8.0 ± 1.8% (values from 4 independent experiments). Addition of high concentrations (50 mM and 100 mM) of L-alanine and further incubation failed to induce a turbidity decrease.
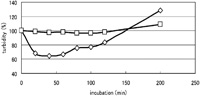 View Details | Fig. 2. Germination of mutant spores of the strain with a trace of CL in LB medium. Purified spores of wild type (◇) and of SDB206 strain (clsA::pMutin4 ywjE::spc ywiE2::neo) with a trace amount of CL (□) were heat activated, suspended in LB medium and germination was monitored by the turbidity fall with Klett-Summerson colorimeter (no. 54 filter) at 37°C. Percentages of initial turbidity are shown. |
Adding AGFK to Tris-HCl buffer was almost as ineffective in inducing a turbidity decrease (Fig. 3B); however, the turbidity decrease of SDB206 spores did gradually start and approached 14.1 ± 4.4% after 60 min (values from 6 independent experiments). The decrease was ca. 35% and ca. 50% after further prolonged incubation for 600 min and 1200 min, respectively. The delay in the decrease was not due to insufficiency of the concentration of AGFK (10 mM) used; addition of much higher concentrations (50 and 100 mM) of AGFK did not speed up the decrease. These results indicate that CL is involved in an early step(s) of germination related to the function of the nutrient receptors. Indeed, the differential effect of CL deficiency in the L-alanine and AGFK experiments, suggests that CL is involved in the functioning of receptors for these germinants. The L-alanine receptor may have a strict requirement for CL, but the requirement of the AGFK receptor may not be so stringent.
 View Details | Fig. 3. Germination of mutant spores with a trace of CL induced with germinant L-alanine (A) and AGFK (B). Purified spores of wild type (◇), of SDB206 (clsA::pMutin4 ywjE1::spc ywiE2::neo) (□) and SDB210 (clsA::pMutin4 ywjE1::spc ywiE2 ::neo ΔpssA::cat) (△) strains with a trace CL, and of SDB211 (ΔpssA::cat) with no phosphatidylethanolamine (○) were heat activated, suspended in Tris-HCl buffer (pH 8.0) containing 10 mM L-alanine or 10 mM each of the combination of asparagine, glucose, fructose and KCl (AGFK). Turbidity was measured with a Klett-Summerson colorimeter (no. 54 filter) at 37°C. Percentages of initial turbidity are shown. The representative data from at least three independent experiments is shown in both A and B. |
Upon exposure to germinants, receptors trigger detectable changes in the dormant spore, in temporal order, which include Ca 2+ and DPA release. The DPA release in the mutant spores with trace CL was to a large extent repressed (15–20% of that of the wild type) after addition of L-alanine or AGFK. On the other hand, DPA is released in large quantities after exposure to a cationic surfactant, dodecylamine, and spores lacking either of, or even all, the known nutrient receptors release DPA well with dodecylamine (Setlow et al., 2003). We were not able to measure the turbidity decrease in this case because dodecylamine induces clumping of spores and this affects turbidity significantly as described by Igarashi et al. (2004). When the mutant spores with a trace amount of CL were exposed to dodecylamine, the DPA release was not repressed, but at a rate comparable to that of the wild type (Fig. 4). These results indicate that the step that CL is involved in is upstream from DPA release, supporting the idea that CL is important to the functioning of the germinant receptors.
 View Details | Fig. 4. Dipicolinic acid release from spores after dodecylamine treatment. Purified spores of wild type (◇), of SDB206 (clsA ::pMutin4 ywjE1::spc ywiE2::neo) (□) and SDB210 (clsA ::pMutin4 ywjE1::spc ywiE2::neo ΔpssA::cat) (△) strains with a trace CL, and of SDB211 (ΔpssA::cat) with no phosphatidylethanolamine (○) were suspended at OD600=1.0 in potassium phosphate buffer (pH 7.4) containing 1 mM dodecylamine. The amount of DPA released after incubation at 37°C was monitored at OD270 and expressed as a percentage of the release after 1h incubation at 95°C. The representative data from three independent experiments is shown. |
What might be the role of CL in the functioning of the germinant receptors in the spore membranes? Two different features of the involvement of CL in the functioning of proteins in cell membranes are known (Dowhan et al., 2004). One is that they glue protein components into higher order supercomplexes as in the case of the yeast mitochondrial respiratory chain, in which CL plays a central role in the association of complexes III and IV into a supercomplex (Zhang et al., 2005). On the other hand, lipids lie as integral components on the surface of proteins or between subunits of multi-subunit complexes. One example of this is the Rhodobacter sphaeroides photosynthetic reaction center, in which tight binding of CL to protein is accomplished by ionic interactions at the lipid head group and by van der Waals interactions at the tail group (McAuley et al., 1999). The differential effect of CL deficiency on spore germination with L-alanine and AGFK (Fig. 3) suggests that the L-alanine receptor has a stricter requirement for CL than the AGFK receptor. These nutrient receptors consist of three components A, B, and C. The A and B components, integral membrane proteins, are assumed to interact to form a complex with their cognate C component, probably a lipoprotein (Hudson et al., 2001; Igarashi and Setlow, 2005). These receptors may thus require CL to fold into the proper conformation in the membranes. CL deficiency may cause a deformation of the receptors or loosen their specific interaction involving the C component in the spore membranes. The AGFK receptor complex may be more deformation tolerant than the L-alanine receptor. This suggestion is supported by the observation that, similarly, the lack of prelipoprotein diacylglycerol transferase (GerF), which causes a defect in the lipid modification of the C component, completely eliminates the function of L-alanine receptor (GerA), which is presumed to be localized in the inner spore membranes (Hudson et al., 2001), but does only moderately and slightly, respectively, impair the function of the AGFK receptors, GerB and GerK (Igarashi et al., 2004).
In summary, we have found that spore membranes of B. subtilis are CL enriched and that spore germination is not induced in mutant spores that contain only a trace amount of CL. This suggests that CL plays a role in an early step of germination, involving the functioning of nutrient receptors in the inner spore membranes (Hudson et al., 2001). Since CL is dispensable in the exponential growth phase of both E. coli and B. subtilis, in the sense that in both species mutants with only a trace amount of or no CL show no significant phenotypic change (Kawai et al., 2004; Kikuchi et al., 2000; Nishijima et al., 1988), although E. coli cells under non-growing conditions do require CL for survival (Hiraoka et al., 1993), these bacteria must be able to replace CL by other anionic phospholipids for indispensable functions during vegetative growth (Matsumoto, 2001). The CL requirement during spore germination is the first conspicuous in vivo evidence that CL is not just used as a generic anionic phospholipid but has a specific function to perform in bacteria. We hope that this finding of a strict requirement for CL during spore germination will open new perspectives for our understanding of the role of CL in bacterial membranes.
We thank Professor Emeritus Isao Shibuya, Yoshito Sadaie, and Hiroshi Matsuzaki for encouragement and helpful discussions. Thanks are also due to Ari Takeuchi and Osamu Makino (Sophia Univ.) for their help in spore disruption. This work was supported in part by Grants-in-Aids for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
|