| Edited by Hideo Shinagawa. Chikashi Shimoda: Corresponding author. E-mail: shimoda@sci.osaka-cu.ac.jp |
The budding yeast Saccharomyces cerevisiae is a model eukaryote amenable to both classical and molecular genetics. Mutational analysis is easily applicable to yeasts in order to define molecular and cellular functions of the respective gene products. The recent advancement of recombinant DNA technology allows insertional, deletion and site-directed mutagenesis, as well as random mutagenesis by PCR. In spite of the availability of these in vitro mutagenesis techniques, we should not overlook the importance of in vivo mutagenesis using chemical reagents and radiation (Lawrence, 1991).
A large subunit of the DNA polymerases δ and ε contains 3’→5’exonuclease activity, which is responsible for removing mismatched nucleotides (for review, Shevelev and Hubscher, 2002). This intriguing task, known as ‘proofreading’, plays an important role for keeping a high fidelity of DNA replication. The S. cerevisiae genes POL3 and POL2 encode the catalytic subunits of DNA polymerases δ and ε, respectively (Morrison et al., 1990; Simon et al., 1991; Araki et al., 1991; for review, Kawasaki and Sugino, 2001). These proofreading domains are highly conserved among eukaryotic DNA polymerases (Morrison et al., 1991). The respective proofreading-deficient mutant alleles, pol3-01 and pol2-4, have been generated by site-directed mutagenesis (Morrison et al., 1993; Morrison and Sugino, 1994). These mutants have proved to be strong mutators that create both forward and reverse mutations (Morrison et al., 1993; Morrison and Sugino, 1994).
S. cerevisiae proliferates most actively at temperatures around 30°C. The upper temperature limit is 38°C for most laboratory strains (Stokes, 1971). Growth properties at high temperatures vary from strain to strain, suggesting that tolerance to high temperature stems from a complex genetic background. Although thermotolerant yeasts have been isolated from tropical habitats (e. g., Thanomsub et al., 2004), mutational improvement of laboratory strains has scarcely been successful. In the present study, we have attempted to create S. cerevisiae mutants by using a proofreading-deficient DNA polymerase. As a result, we have obtained thermotolerant mutants that can grow at 40°C. Here we report the procedure to obtain these mutants, together with their physiological properties and genetic background.
The S. cerevisiae strains used in this study are listed in Table 1. Yeast cells were grown in yeast extract/polypeptone/dextrose complete medium (YPD). Synthetic media (SD and SC) supplemented with appropriate nutrients were used to assess nutritional requirements. The chemical composition of these media and concentrations of supplements have been described previously (Amberg et al., 2005). Sporulation was induced in 1% potassium acetate medium (Amberg et al., 2005). Total cells were counted by a particle count analyzer (CDA-500, Sysmex Co. Kobe). Viable cells were counted as colony-forming units after incubation for at least 3 days on YPD plates.
 View Details | Table 1. Strain list |
Diploid strains were incubated on solid sporulation medium at 30°C for 2 days. Asci were treated with 0.1 mg/ml of zymolyase T20 (Seikagaku Kogyo Co., Tokyo) for 10 min at room temperature to disrupt ascal walls. Free spores were separated on a thin YPD agar plate by micromanipulation and cultured for 2 days at 30°C. Haploid segregants were scored for nutritional requirement and colony-forming ability at different temperatures.
Yeast cells were fixed with 3% formaldehyde at room temperature. Cellular F-actin was stained with rhodamine-conjugated phalloidine (0.1 μg/ml), and DNA with 4’,6-diamidino-2-phenylindole (DAPI) at 1 μg/ml. Stained cells were observed under a fluorescence microscope (Olympus BX50, Olympus Co., Tokyo) (Amberg et al., 2005). Cell morphology was also observed by differential interference contrast (DIC) microscopy (Olympus BX51, Olympus Co., Tokyo).
We attempted to isolate high temperature-oriented and tolerant (hot) mutants from proofreading-deficient mutator strains of S. cerevisiae to assess the effectiveness of these mutators. We considered that genetic and physiological analysis of the thermotolerant hot mutants would provide clues to the genetic background of thermotolerance. Two isogenic strains, AMY52-3D (POL3) and AMY128-1 (a proofreading-deficient mutant of DNA polymerase δ, pol3-01), were used as parental strains. These haploid strains grew at 37°C, but showed severe growth retardation at temperatures higher than 38°C. Frozen stocks were regrown on YPD plates and single colonies were isolated. Cells were inoculated into YPD liquid medium at cell density of about 104 cells/ml. For each strain, five batch cultures were shaken at 30°C for 3 days. Aliquots of these stationary-phase cultures were spread on YPD plates, and then incubated at either 30°C, 38.5°C or 40°C. The pol3-01 culture produced colonies at a frequency of 2.46 × 10–5 at 38.5°C, but no colonies were formed at 40°C (Table 2). The POL3 strain formed no colonies even at 38.5°C (Table 2). These results indicate that the pol3-01 mutator is effective in producing thermotorelant (hot) mutants. Using the same procedure, the medial thermotolerant mutants were obtained from another pol3-01 parent, BYD12-5A. Genetic analysis of one of the typical hot mutants (RM1) defined a single recessive mutation, hot1-1, as described below. Growth of RM1 and its parent at different temperatures is shown in Fig. 1A. Only RM1 grew at 38.5°C.
 View Details | Table 2. Frequency of high temperature-resistant mutants in pol3-01 strains |
 View Details | Fig. 1. Growth of parental, thermotolerant, and hybrid diploid strains at high temperature. (A) A medial thermotolerant mutant RM1, its parental strain BYD12-5A and the hybrid diploid strain BYD15 were used. Single colonies were picked and precultured for 2 days on YPD. A few cells were then inoculated onto YPD plates and incubated at either 30°C, 38.5°C or 40°C for 2 days. (B) A parental strain AMY128-1 and its derivative BYH10 were used as high-temperature-sensitive control strains. The thermotolerant mutants RY1 and RX7 were independently isolated from AMY128-1 and BYH10, respectively. For other strains, see Table 1. Cells were incubated at either 30°C or 40°C for 2 days. |
To obtain mutants that could grow at higher temperatures, the pol3-01 colonies grown at 38.5°C were collected and then further incubated on YPD plates at 39°C for 5 days. Several colonies grew on these plates. Cells were subsequently collected from these colonies and inoculated on YPD plates, which were then incubated at 40°C. A few small colonies appeared after 3 days and continued to grow slowly (colony diameter, ca. 1 mm) for another 3 days. Seven single colonies were subsequently isolated (RY1–RY7) as strong thermotolerant mutants for further inspection. These hot mutants formed tiny colonies (< 0.5 mm) even at 41°C, but no visible colonies were observed at 42°C (data not shown). Similar isolation procedures also gave strong hot mutants (RX1–RX7) from another parental strain, BYD10 (pol3-01). On the other hand, POL3 strains (AMY52-3D and BYH1) multiplied very poorly at 38.5°C on YPD plates. Cells were heavily inoculated onto YPD plates and subjected to the stepwise elevation of temperatures as described above. This procedure, however, did not produce visible colonies on YPD plates at 40°C. We thus conclude that pol3-01 mutator is effective in generating strong thermotolerant mutants. Growth of the parental and mutant strains on YPD plates at 30°C and 40°C is shown in Fig. 1B. Only thermotolerant hot mutants grew at 40°C.
The growth properties of the thermotolerant mutant RY1 were quantitatively studied in comparison to those of its parental strain (AMY128-1). Cells grown to stationary phase at 34°C for 2 days on YPD medium were suspended in liquid YPD medium at a cell density of ca. 4 × 105 cells/ml (OD530 nm = 0.1). Cultures were incubated with shaking at 30, 37, 38, 39 and 40°C. The yields of each culture were measured after 48 hr with a spectrophotometer (JASCO V-530). Growth of the parent was better than that of the hot mutant RY1 at 30°C, whereas RY1 grew better than the parent at temperatures higher than 37°C (Fig. 2). The parental strain showed poor or no growth at temperatures higher than 38°C (Fig. 2).
 View Details | Fig. 2. Comparison of the growth capacity of the thermotolerant mutant and its parental strain at different incubation temperatures. Cells of AMY128-1 and RY1 were precultured on YPD plates at 34°C and inoculated into fresh YPD liquid at OD530 = 0.1 (~4 × 105 cells/ml). After 48 hr, the optical density of each culture was measured and the increase in OD530 was calculated. Triplicate cultures were run for each strain. The mean fold increase ± standard deviation (vertical bars) is shown. Open columns, AMY128-1 (parental strain); filled columns, RY1. |
Time-course studies are summarized in Fig. 3A. At 30°C, the growth rate of the RY1 mutant during the log phase was slightly lower than that of the parent. At 40°C, RY1 steadily proliferated even after 50 hr, whereas the parent ceased growing after 8 hr of incubation.
 View Details | Fig. 3. Growth properties of thermotolerant mutant at high incubation temperature. (A) Growth curve. Cells of AMY128-1 (parent) and RY1 (mutant) were precultured on YPD plates and inoculated into YPD liquid medium. Cultures were shaken at 30°C or 40°C. At intervals, OD530nm was measured. Filled squares, AMY128-1; open squares, RY1. (B) Cell morphology. After incubation at 40°C for 24 hr, aliquots of the cultures were taken and morphologically inspected under a DIC microscope. Shrunk cytoplasm in AMY128-1 is indicated by an arrow. Bar, 10 μm. (C) Viability after incubation for 48 hr at 40°C. Aliquots of cultures containing 1 × 103–1 × 105 cells were spread on YPD plates, and then incubated at 30°C for 2 days. |
Exposure of the parental culture to 40°C resulted in shrinkage of the cytoplasm, whereas cell morphology appeared to be normal in the hot mutant (Fig. 3B). Staining of the parental cells with a DNA probe (DAPI) revealed that the fluorescent signal was not restricted to the chromatin region, but was spread over the cytoplasm (data not shown). In addition, cell polarity, as assessed by the localization of actin patches, was completely lost in parental cells incubated at 40°C (data not shown). By contrast, nuclear chromatin regions were clearly maintained and actin patches were localized in a polarized fashion in most RY1 cells at 40°C. These observations indicate that extremely high incubation temperatures disrupt basic cell structures and cell polarity in parental cells.
The aberrant morphology of parental cells suggested that high temperature reduces cell viability. To test this, a portion of the culture after incubation for 48 hr at 40°C was spread on YPD plates, which were then incubated at 30°C for 5 days. As shown in Fig. 3C, the viability of the parental strain was greatly reduced (< 10–3), whereas RY1 maintained viability at a level of ~30%.
As mentioned above, the hot mutant RY1 showed proliferation at 40°C. We therefore compared the heat shock resistance of RY1 with that of the parent. Suspensions of stationary-phase cells in sterilized, deionized water were exposed to 55°C for 30 min and were measured their viability at 30°C. The viability of RY1 was rather lower than that of the parent (data not shown), suggesting that ability of RY1 to proliferate at elevated temperatures was not due to a higher degree of heat shock resistance.
We carried out genetic analysis of the medial and strong hot mutants isolated to obtain clues to how thermotolerance is genetically controlled. First, the medial thermotolerant mutant RM1 was crossed with BYD13-4A to obtain hybrid diploid strains. None of the diploids was able to grow at 38.5°C, indicating that the hot mutation is recessive. One of the hybrids, BYD15, was then subjected to tetrad analysis in which haploid meiotic segregants were scored for their ability to grow at 38.5°C. The thermotolerance mostly segregated regularly, indicating that the ability to grow at 38.5°C is controlled by a single recessive mutation, designated here as ‘hot1-1’. As shown in Table 3, the linkage relationship between hot1 and two centromere-linked markers, leu2 (Chr3) and trp1 (Chr4), strongly suggested that the hot1 gene is linked with a centromere (< 13.5 cM). Thus, the hot1-1 single mutation confers medial thermotolerance on S. cerevisiae.
 View Details | Table 3. Genetic analysis of the hot1-1 mutation |
Next, similar meiotic analysis was carried out with the strong hot mutant RY1. We crossed RY1 with a wild-type MATa strain (BYH10) to obtain a hybrid diploid strain, BYD11. As a control, the parental strain (AMY128-1) was crossed with BYH10 to isolate a diploid strain, BYD10. Neither BYD10 nor BYD11 could form colonies at 40°C (Fig. 1B), suggesting that the mutation was recessive. Both BYD10 and BYD11 were sporulated, and asci were dissected with a micromanipulator. As the germination efficiency was low (~20%) in both diploid strains, germinated spores were analyzed in a random fashion. Meiotic segregation of genetic markers was scored (Table 4). The auxotrophic markers appeared at a frequency of roughly 50% among haploid spore clones, indicating that these reference markers segregated regularly. By contrast, the frequency of the thermotolerant segregants was only 18% of the spore clones scored. We conclude that this phenotype is mediated by multiple mutations, at least one of which is recessive. Another strong thermotolerant mutant RX7 was crossed with BYH1 (POL3) to generate BYD17 (Table 1). Tetrad analysis also gave the same conclusion that RX7 harbored multiple hot mutations (data not shown), at least one of which was recessive (Fig. 1B). A portion of the thermotolerant segregants harbored the wild type POL3 allele, indicating that the pol3-01 allele did not closely link with any hot alleles. Generally, the pol3-01 mutator could be meiotically eliminated from the desired mutants by crossing with a POL3 strain.
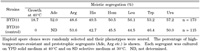 View Details | Table 4. Genetic analysis of the thermotolerant phenotype |
The hot1 mutation resulted in medial thermotolerance (growth at 38.5°C). Strong tolerance to higher temperatures (40°C) required multiple mutations. By gradually raising the incubation temperature up to 40°C, a pol3-01 mutant harboring a proofreading-deficient DNA polymerase δ accumulated mutations increasing thermotolerance. Elevated temperatures may act as an efficient selective pressure. Parallel attempts (three times) with a chemical mutagen, ethyl methanesulfonate (EMS), produced very few medial thermotolerant mutants that formed colonies only at 39°C, meaning that EMS was less efficient as compared with the pol3-01 mutator. Using the pol3-01 mutator, we also succeeded in obtaining S. cerevisiae mutants that were resistant to high concentrations of salts (C. Shimoda, unpublished data). These findings imply that a proofreading-deficient DNA polymerase δ variant is a potent mutator for obtaining useful yeast mutants. Despite its high mutagenic activity, pol3-01 reduces viability only slightly if another replication DNA polymerase ε (Pol2) is intact. A proofreading-deficient mutant of DNA polymerase ε (pol2-4) has been also found to show a mutator phenotype (Morrison et al., 1991). Haploid pol2-4 pol3-01 double mutants are lethal (Morrison and Sugino, 1994). We found that pol2-4 is less efficient at inducing thermotolerant mutants (data not shown), consistent with its weak mutator phenotype (Morrison et al., 1991).
The heat shock resistance in yeast is well explained by the induction of heat-shock proteins (Lindquist and Kim, 1996), accumulation of trehalose (de Virgilio et al., 1994) and changes in lipid composition of cellular membranes (Swan and Watson, 1997; Swan and Watson, 1999), whereas the mechanism underlying thermotolerance (the ability to grow at sublethal high temperatures) is not well understood. Extreme thermotolerant and thermophilic yeasts have been isolated from tropical habitats. Owing to diverse phylogenetic relationships, it is difficult to find significant biochemical and genetical differences between these thermotolerant/thermophilic yeasts and laboratory yeasts. Comparative studies of thermotolerant mutants with the parental S. cerevisiae strains might provide important information on thermotolerance. Our present study has demonstrated that a proofreading-deficient mutator is an efficient tool for isolating thermotolerant mutants and has defined the gene hot1, as well as a few additional hot genes that remain to be genetically defined. Characterization of these hot genes by cloning and sequencing will shed light on the molecular mechanism of thermotolerance in yeast.
We thank Masayo Morita and Shikiko Murakami for their excellent technical assistance.
|