| Edited by Etsuko Matsuura. Masayoshi Watada: Corresponding author. E-mail: watada@mserv.sci.ehime-u.ac.jp |
The Drosophila auraria species complex, distributed mainly in Japan, China and Korea, is a part of the montium species subgroup, the largest known subgroup of the melanogaster species group (Lemeunier et al., 1986; Kimura, 1987). Bock and Wheeler (1972) first described D. auraria, D. biauraria, D. triauraria and D. quadraria and placed them into the auraria complex. Subsequently, Kimura (1983) reported D. subauraria and proposed that it is a new species in the complex based on similarity to D. biauraria. The external morphology of these five species is similar (for example, they are characterized most notably by males with white heads) and morphological differences are observed only in their genitalia. The sixth member of the complex, D. rufa, has a characteristic black line in its both sides of thorax and was classified as a member of the auraria complex based on the results of two dimensional electrophoretic analysis (Ohnishi et al., 1983) and on the results of mating experiments within the montium species subgroup (Kim et al., 1989).
The members of the auraria complex have been well studied with regard to reproductive isolation (Kurokawa, 1960; Kurokawa et al., 1982; Kim et al., 1989), flight activities (Kimura, 1992), ability of diapauses (Kimura et al., 1993), courtship songs (Tomaru and Oguma, 1994a, 1994b; Tomaru et al., 1998), and cold tolerance (Ohtsu et al., 1998). For many of these characteristics, researchers have observed differences among species, with the exception of D. triauraria and D. quadraria, which appear to be identical.
Previously, phylogenetic studies of the auraria complex have not usually included three endemic species of the montium subgroup in Japan, two of which are considered to be closely related to members of the auraria complex based on morphological data. D. asahinai is an endemic species found only in Amami-oshima and Tokunoshima, and has a black line on its thorax like D. rufa (Okada, 1964). D. yuwanensis is also found in Amami-oshima and has been recorded as a species closely related to D. asahinai (Kim and Okada, 1988). However, this species now appears to be the same as D. asahinai rather than a distinct species (M. T. Kimura, personal communication). D. lacteicornis is also an endemic species, found only in Okinawa, Ishigakijima, and Iriomotejima (Okada, 1965). In addition to having a black line on the thorax, D. lacteicornis males also have white antennae. The third endemic species, D. pectinifera, is found only in the Ogasawara (Bonin) islands and its external morphology is similar to D. auraria, D. rufa and D. kikkawai (Wheeler and Takada, 1964). However, the sex combs of D. pectinifera are different from those of the auraria complex members. Moreover, its phylogenetic position has been unclear, as there have been no reports on the reproductive isolation, behavior, or molecular phylogeny of D. pectinifera.
There are many discrepancies among the auraria complex phylogenetic trees reported in previous studies. Perhaps the most peculiar case is the position of D. lacteicornis based on two dimensional electrophoretic analysis, which positioned D. lacteicornis far from the auraria species complex in the montium species subgroup (Ohnishi et al., 1983). Recently, Zhang et al. (2003) reported the phylogenetic relationship within the montium species subgroup based on the comparison of the multiple alpha-amylase genes. Their results suggested that D. rufa belongs to a lineage separated from the other five species in the auraria complex. However, the phylogeny of the auraria complex remains unclear because the researchers used Amylase genes for their study. Moreover, previous phylogenetic studies have typically relied on analysis of only one strain from each species. And thus, some of the differences detected in the analyses might not be the result of species-specific mutations but instead of the strain-specific mutations. In order to sort out the phylogenetic relationships among these closely related species, it is important to estimate the amount of both intraspecific and interspecific differences.
The internal transcribed spacer (ITS) of the ribosomal DNA is a rapidly evolving region. It is not translated and there are usually hundreds of copies per genome. For these reasons, ITS is recognized as one of the more useful tools available for the study of phylogenetic relationships among closely related species. In Drosophila, Schlötterer et al. (1994) analyzed relationships among members of the melanogaster species subgroup using the ITS region and reported that this region was useful because there were many differences within the ITS region among species. In addition to interspecific variation, both intra individual and intraspecific variation was found in ITS sequences of mosquitoes (Wesson et al., 1992; Paskewitz et al., 1993; McLain et al., 1995).
In Drosophila, evolutionary divergence of COII mitochondrial sequences have been well investigated in the obscura species group (Beckenbach et al., 1993; Gleason et al., 1997) and the saltans species group (O’Grady et al. 1998). In addition, sequence diversity in COII was enough to reveal the phylogenetic relationships among closely related species in these groups. Schawaroch (2002) and O’Grady and Kidwell (2002) used COII sequences and two nuclear genes for phylogenetic study of several melanogaster species groups, including the montium species subgroup. However, because few members of the auraria complex were included in the analyses, the authors did not discuss the phylogenetic relationships among its members.
In this study, we examined both intraspecific and interspecific variation of nuclear ITS1 and mitochondrial COII sequences among ten species of the montium species subgroup. In addition, we report the phylogenetic relationships among members of the auraria species complex and allied species as determined by the analysis of ITS1 and COII sequences.
Twenty-seven strains of ten species in the montium species subgroup were used in this study (Table 1). In the survey of intra- and interspecific variation, at least two strains collected from geographically separated localities were used for each species but one (Fig. 1). Since only single female of D. quadraria had been collected in Taiwan, we used only one strain (Q strain) for D. quadraria from University of Tsukuba, same as the original stock kept in the US Stock Center. D. punjabiensis, which belongs to the jambulina species complex, was used as an outgroup.
 View Details | Fig. 1. Collection localities of the original stocks of the auraria species complex and allied species used in this study. |
DNA was extracted from five females of each strain using DNAzol (Invitrogen) and following the supplier’s protocol. To amplify the full length of ITS1(680–763bp), primers 7246 (5’-GCTGCGTTCTTCATCGAC-3’) and 7247 (5’-CGTAACAAGGTTTCCGTAGG-3’) were designed based on a highly conserved region of 18S and 5.8S ribosomal DNA (Tautz et al., 1988; Schlötterer et al., 1994). The primers CO2-a (5’-ATATGGCAGATTAGTGCAA-3’) and CO2-b (5’-TTGCTTTCAGTCATCTAATG-3’) were used to amplify a region of the mitochondrial COII from the start codon to position 645 (the full length of the COII open reading frame is 688 bp). The primer design was based on the mitochondrial DNA sequence of D. melanogaster (Beckenbach et al., 1993). PCR amplification was carried out with AmpliTaq DNA polymerase (Applied Biosystems) under standard conditions. Thermal cycle profiles were as recommended by the supplier.
ITS1 PCR products were cloned into the pCR2.0 vector using the TOPO TA-cloning Kit (Invitrogen). DNA from several independent clones was extracted using the QIAprep Mini Kit (QIAGEN). ITS1 was sequenced in both the 5’ and 3’ orientations using the standard primers T7 and M13 reverse. Amplified COII products were diluted to 1 ng/μl and used as sequencing template after their sizes were determined. Sequencing of COII was done using the same primers used for PCR amplification. All sequence reactions were done with the BigDye Terminator Cycle Sequencing Kit (ABI) and using an ABI PRISM 310 Genetic Analyzer. The 72 ITS1 sequences and the 27 partial COII sequences obtained in this study were deposited in the DDBJ database (Table 1).
 View Details | Table 1. List of species and strains of the montium species subgroup used in this study, length of ITS1 and accession No. |
Phylogenetic analysis of sequence data was performed using the MEGA3 software package (Kumar et al., 2004). ITS1 and COII sequences were aligned automatically using the ClustalW program (Thompson et al., 1994) that is included in MEGA3. Because many gaps were observed in aligned ITS1 sequences, we corrected the alignments manually. Aligned sequences were used to construct phylogenetic trees using both the neighbor-joining (NJ) method (Saitou and Nei, 1987) and the maximum parsimony (MP) method. The nucleotide distances used for the NJ trees were estimated by Kimura’s two-parameter method (Kimura, 1980). Bootstrap values were obtained after 1000 replications. Because many gaps were present in the aligned ITS1 sequences, gaps were treated by pairwise deletion method for NJ trees.
A total of 72 individually cloned ITS1 sequences from 27 strains of ten species of the montium species subgroup were used in the analysis (Table 1). Small size variations attributable to indel (insertion/deletion) mutations were observed among most strains and species. Comparison between ITS1 sequences within a given species revealed that nucleotide length polymorphisms were detected in all strains except D. auraria (UW91). Single nucleotide substitutions and indels were distributed randomly across the ITS1 region analyzed.
Based on the alignment of ITS1 sequences, eight species (all but D. pectinifera) can be separated into two groups, mainly on the basis of indel mutations and single nucleotide substitutions. The first group consists of the following five species: D. auraria, D. triauraria, D. quadraria, D. biauraria, and D. subauraria (Group A). The second group consists of D. rufa, D. asahinai, and D. lacteicornis (Group R). The sequences of Group A and Group R member species have high sequence similarity to one another within the group (>96%). However, the sequence similarity between Group A and Group R species was not high, as similarity was reduced to an average of 82%. In the melanogaster species subgroup, the ITS1 region consists of a highly diverged 5’ half and a conserved 3’ half (Schlötterer et al., 1994). Our results similarly suggest that, the 5’ half sequence was highly conserved within both Group A and R, as indel mutations with more than five nucleotide substitutions were not found in this region. Similarities were, on average, low between D. pectinifera and Group A members (48%) or Group R members (47%) due to the presence of large, unique indel mutations and substitutions in D. pectinifera. These differences were detected both in the 5’ and 3’ halves of the ITS sequence.
A summary of variable sites for Group A (with ITS1 of D. auraria used as the reference sequence) is shown in Table 2. There were 57 variable sites in 32 clones of five Group A species. Of these sites, 24 sites appear to reveal intraspecific variation (variable site within a species) and 22 sites appear to be single nucleotide substitutions that differ between species. In addition to species-specific substitutions and substitutions common to D. biauraria and D. subauraria, three insertions and five deletions were also observed. D. biauraria and D. subauraria were separated from other Group A species by the nine common substitutions. There were three variable indel mutations among the five species of Group A. Interspecific differences were not found among the ITS1 sequences of D. auraria, D. triauraria and D. quadraria, and these species could not be distinguished based on ITS1 sequences alone.
 View Details | Table 2. Summary of variable sites in ITS1 sequences of Group A species |
For Group R, the D. rufa ITS1 sequence was used as a reference sequence and a summary of variable sites is shown in Table 3. There were 59 variable sites in 25 clones of the three species in Group R. Of these, 45 sites were attributable to intraspecific variation and 11 sites appear to be single nucleotide substitutions that are different among the three species. In the AB243347 D. rufa clone, many intraspecific variations were found and a stretch of five A residues was substituted for TTGCC at position 79–83. There were two sites of insertion and two sites of deletion in D. asahinai and D. lacteicornis. In clones of D. lacteicornis, seven nucleotides (AAAAGAA) were substituted for 20 nucleotides (TTATTTAATTTTTTTAATTT) at the middle of the sequence of the clone AB243326 and 26 nucleotides were deleted at about 160 downstream from 5’ end of the sequence of the clone AB243328. Interspecific variation was higher than intraspecific variation among the three species, which could clearly be distinguished based on only the ITS1 sequences.
 View Details | Table 3. Summary of variable sites in ITS1 sequences of Group R species |
Mitochondrial COII sequences were analyzed from the same 27 strains in Table 1. Nucleotide substitutions detected among nine species are shown in Table 4. Intraspecific variation in COII was detected in most of the species, with exceptions of D. quadraria and D. biauraria. Other Group A species had intraspecific variation at one site. By contrast, Group R species showed intraspecific variation at five to seven sites. In addition, D. pectinifera showed intraspecific variation at five sites (as many as those detected among Group R species). Of the total number of substitutions, 10.8%, 1.2%, and 88% occurred at the first, second, and third position of codons, respectively.
 View Details | Table 4. Summary of variable sites in COII partial sequences of the ten species of the montium species subgroup |
Based on the analysis of the COII sequences, Group A can be further subdivided into two units, with one unit consisting of D. biauraria and D. subauraria, which have three common substitutions, and the other unit consisting of D. auraria, D. triauraria and D. quadraria, which have five common substitutions. D. subauraria had a single unique substitution (at position 390) that distinguished it from the other four species. D. quadraria had a unique substitution (at position 324) and a common substitution (at position 462) with D. biauraria and D. lacteicornis. Group R species share the same 16 substitutions in the COII sequences relative to Group A. Moreover, D. lacteicornis can be distinguished from D. rufa and D. asahinai on the basis of three substitutions. Furthermore, D. lacteicornis had only one unique substitution (at position 234), D. rufa had six unique substitutions, and D. asahinai had one unique substitution (at position 513). Within Group R, all three species could clearly be distinguished based on the COII sequences.
Nine substitutions were shared between D. pectinifera and the auraria complex and ten unique substitutions existed in the sequences of D. pectinifera (Table 4). As D. pectinifera has many unique substitutions in the COII sequence, D. pectinifera appears to be distinct from Group A or Group R.
Phylogenetic trees based on the ITS1 and COII sequence data are shown in Fig. 2 and Fig. 3, respectively. As both the NJ and MP trees from a given dataset have the same topology, only the NJ trees are shown. The ITS1 and COII trees have an identical topology besides the taxonomic position of D. quadraria. Different from the results in the ITS1 tree, this species was located outside the D. auraria and D. triauraria cluster in the COII tree because of a species-specific substitution at two sites (Table 4). As mentioned for the sequence data, the two phylogenetic trees also show that the nine species analyzed can be divided into two taxonomic groups with high bootstrap values (>84). In Group A, D. biauraria and D. subauraria formed a small cluster distinct from the other species. Intraspecific variation in Group A is very low in both the ITS1 and COII trees. In this study, we used D. auraria strains from four distant locations and D. triauraria strains from three distant locations. Despite these samplings, there is no distinction between D. auraria and D. triauraria in the ITS1 and COII trees.
 View Details | Fig. 2. Neighbor-joining tree for ITS1 among the auraria complex members and allied species. The number of clones that were sequenced for a given strain is shown in parenthesis after the strain name. Bootstrap values are indicated on the branches with the values from MP method in parentheses. Values less than 50% are not shown. |
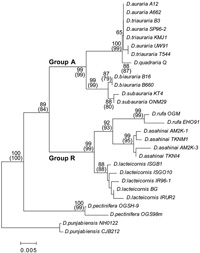 View Details | Fig. 3. Neighbor-joining tree for COII among the auraria complex members and allied species. Bootstrap values are as shown for Fig. 2. |
The phylogenetic trees also revealed that two endemic species, D. asahinai and D. lacteicornis, are closely related to D. rufa and can be classified as members of the auraria species complex. D. lacteicornis is separated from D. rufa and D. asahinai in the cluster. In Group R, intraspecific variation was relatively large for both the ITS1 and the COII trees. Nevertheless, interspecific variation was much larger among the three Group R species. D. pectinifera belongs to a distinct cluster from D. punjabiensis and the other eight species of the auraria complex, suggesting that D. pectinifera is a related species best categorized as outside the auraria complex.
In the present study, we used at least two geographically distant strains for each species, with exception of D. quadraria, for a molecular phylogenetic analysis of DNA sequences from the nuclear ITS1 and mitochondrial COII. By examining both intraspecific and interspecific variation, we revealed interesting relationships among members of the auraria species complex and their allied species. Nine of the ten species analyzed can be grouped into two groups, which we name Group A and R.
Comparison of the ITS1 and COII sequences among geographical strains revealed the presence of intraspecific variation in most of the species studied. Intraspecific variation in Group A was low among species in both the ITS1 and COII sequences. Interspecific variation in ITS1 was of the same magnitude as intraspecific variation among D. auraria, D. triauraria, and D. quadraria, and intraspecific variation of COII sequences in D. auraria and D. triauraria were almost the same as the interspecific variation of these species. Previous studies reported interspecific variation based on one isofemale strain of each member of the auraria complex and did not take intraspecific variation into consideration. Here, we demonstrated the importance of surveying both intraspecific and interspecific variation in order to elucidate the phylogenetic relationships among the closely related species.
Some amount of intra-individual variation at ITS1 was reported in a study of mosquitoes (Wesson et al., 1992). By contrast, the level of intraspecific variation in ITS1 among the montium species subgroup was low, particularly within Group A. Although interspecific variation was small within each group, a greater degree of interspecific variation was detected between groups. We have sequenced ITS1 in D. baimaii, D. mayri and D. kikkawai (unpublished data), which are closely related to members of the auraria complex, (Ohnishi and Watanabe, 1984; Zhang et al., 2003). Similarity of the sequences between those three species and members of the auraria complex is very low, which is attributable to the presence of numerous indel mutations that prevent alignment of the sequences (data not shown). These results suggest that detection and comparison of indel mutations in ITS1 provide reasonable criteria for distinguishing species complexes. Based on the ITS1 data, D. pectinifera cannot be included in the auraria complex and instead, appears to be a closely related species outside the complex.
Intraspecific variation at COII was detected in the nine species (all but D. biauraria) and interspecific variation at COII was found in all but D. auraria and D. triauraria. There was no difference in the COII sequences from D. auraria and D. triauraria, even at a typically polymorphic site, position 117 (Table 4). In the COII sequences, D. quadraria had only one unique substitution (at position 324) and was distinct from D. auraria and D. triauraria (Fig. 3). However, the same substitution (at position 324 of COII) was found in the Chinese D. triauraria strain (O’Grady and Kidwell 2002), suggesting that species-specific variation does not exist among COII and ITS1 sequences from D. auraria, D. triauraria and D. quadraria.
The analysis revealed that the eight species can be separated into two groups, Group A and R (Fig. 2 and Fig. 3). Group A consisted of D. auraria, D. triauraria, D. quadraria, D. biauraria, and D. subauraria; D. rufa formed the other taxonomic group, Group R, which also included D. asahinai and D. lacteicornis. ITS1 sequences showed high similarity within each group but the similarity between the two groups was not as robust. D. rufa was previously considered a member of the auraria complex based on the results of two dimensional electrophoretic analysis and allozyme data (Ohnishi et al., 1983; Ohnishi and Watanabe, 1984). The results of molecular phylogenetic studies using alpha-amylase (Zhang et al., 2003) and a restriction enzymatic study of mitochondrial DNA (Kim et al., 1993) further suggested that D. rufa formed a cluster with D. lacteicornis or D. yuwanensis. However, amylase genes were not suitable for the study of the closely related species, because phylogenetic studies using alpha-amylase formed a cluster of D. triauraria, D. quadraria and D. subauraria. Although a diagnostic difference between D. auraria and D. triauraria was not observed at the ITS1 and COII, the results of phylogenic study of the auraria complex presented here are concordant with the results of comparative study of morphology, reproductive isolation and physiology (Kurokawa et al., 1982; Kimura, 1983; Kimura et al., 1993; Tomaru and Oguma, 1994a; Ohtsu et al., 1998; Tomaru et al., 1998).
In most previous studies, molecular analyses were usually based on only one strain for each species of the auraria species complex. As a result, it was difficult to distinguish intraspecific variation from interspecific variation in these studies. In the present study, we obtained a reasonable estimate of intraspecific and interspecific variation at ITS1 and COII. The finding that interspecific variation was of the same magnitude as intraspecific variation among D. auraria, D. triauraria, and D. quadraria in ITS1 and COII sequences was unexpected, as previous molecular phylogenetic studies detected differences between the species. Moreover, we did not find a diagnostic difference between D. auraria and D. triauraria at the ITS1 and COII despite the fact that reproductive isolation and behavioral differences between the two species have previously been reported (Kurokawa et al., 1982; Tomaru and Oguma, 1994a, 1994b; Tomaru et al., 1998).
This lack of concordance between the results of our molecular study and those of previous reproductive and behavioral studies is intriguing. There are at least two explanations. One explanation is that speciation between D. auraria and D. triauraria occurred quite recently and that genes involved behavioral or physiological differences in these species evolved more rapidly than ITS1 and COII regions. Unfortunately, it is currently impossible to compare genetic divergence of physiological and behavioral traits for these species. Another possible explanation is that natural hybridization between the two species has occurred. Indeed, laboratory hybridization between D. auraria and D. triauraria has been reported (Kurokawa, 1960; Kim et al., 1989). Moreover, Kurokawa (1967) reported that hybrid males with a distinctive phallic organ morphology were found at two distant natural locations where D. auraria and D. triauraria coexist. In addition, one of the authors (M.W.) and Dr. M. T. Kimura (personal communication) recently found males that appear to be D. auraria and D. triauraria hybrids among the offspring of isofemale lines established from females that mated in the wild. These results support the second explanation, and hybridization is sufficient to explain why no significant difference was observed in the ITS1 sequences of D. auraria and D. triauraria.
D. quadraria had been reported to be a member of the auraria species complex (Bock and Wheeler, 1972). The external morphology of this species is quite similar to D. triauraria, with a slight difference between the two species detectable in the male aedeagus. Significant differences between the species in courtship songs (Tomaru and Oguma, 1994a, 1994b) or reproductive isolation (Kurokawa et al., 1982; Kim et al., 1989) have not been reported. Watada and Ito (1999) recorded D. triauraria from Kumejima and suggest that D. triauraria might be a founder of D. quadraria, as only one female was found in Taiwan. The results of our analysis of nuclear ITS1 support this idea but the analysis of mitochondrial COII sequences suggest instead that D. quadraria is outside a cluster that includes D. auraria and D. triauraria (Fig. 3). D. quadraria had only one unique substitution (at position 324) and, in addition, D. quadraria had one substitution (at position 462) that was found also in D. biauraria and D. lacteicornis but not in other species (Table 4). Interestingly, the same substitution (at position 324 of COII) was found in the Chinese D. triauraria strain (O’Grady and Kidwell 2002). In addition, the morphology of the aedeagus in the Chinese D. triauraria strain was intermediate between that of D. quadraria and Kumejima strains of D. triauraria (M. T. Kimura, personal communication). Thus, the D. quadraria in Taiwan may be a descendant of a founder from Chinese populations of D. triauraria.
Wheeler and Takada (1964) described the external morphology of D. pectinifera as similar to that of D. auraria, D. rufa and D.kikkawai. However, D. pectinifera has a different number of sex combs as compared with members of the auraria complex (Fig. 4). The tooth number of the sex combs of D. pectinifera was 60 (55–65) on metatarsi and 50 (45–65) on the second segment, or 2.4–2.8 times more than that is observed for members of the auraria complex (unpublished data). In a comparative analysis of ITS1, D. pectinifera was more similar to members of the auraria complex than to D. punjabiensis, a complex reported as closely related to the auraria complex based on mating experiments (Kim et al., 1989) and molecular phylogenetic study (Ohnishi et al., 1983). Unique sequences existed not only in the highly diverged 5’ half of D. pectinifera ITS1 but also in the highly conserved 3’ half. Overall, D. pectinifera was not recognized as a member of the auraria complex in our analysis for ITS1 (Fig. 2).
 View Details | Fig. 4. Sex combs from a Drosophila pectinifera male (A) and from a D. auraria male (B). A sex comb of D. pectinifera is composed of densely packed fine teeth in upper portion of metatarsal comb and entire portion of second tarsal segment. |
Based on the mitochondrial COII sequence, it was also shown that D. pectinifera belongs to a distinct cluster (Fig. 3). Beckenbach et al. (1993) and Gleason et al. (1997) reported that a strong bias for transitional substitutions was observed between closely related species in the obscura species group and that the bias was lost between more distantly related species. The transition/transversion ratio between D. pectinifera and members of the auraria complex species is low, as D. pectinifera has six unique transversions in COII sequence. This result further suggests that D. pectinifera is not a member of the auraria complex.
D. pectinifera is an endemic species found only in the Ogasawara islands consisted primarily of two small islands, Chichijima (24 km2) and Hahajima (21 km2). This species is not a dominant species on the Ogasawara islands and its population size seems to be very small (Yamamoto et al., 1985), particularly on Chichijima. In small populations like this, the bottleneck effect is likely to be significant, and may lead to rapid evolution of the genome. Speciation of D. pectinifera on the Ogasawara islands seems to be more recent than what is revealed by molecular phylogenetic analysis based on the sequences of ITS1 and COII sequences, because interspecific crosses were observed from the mating experiment between D. lacteicornis females and D. pectinifera males and in the reciprocal crosses (unpublished data). Geologically, the Ogasawara islands separated from the Nansei islands about 15 million years ago (Sibuet et al., 2002) and have been isolated since then. It appears that a large change in the number of teeth present on the sex combs of D. pectinifera took place during or after speciation, as no other montium subgroup species has such a large number of teeth on the sex combs. In Taiwan and other areas of Asia, there are other species of the montium species subgroup, such as D. trapezifrons, that have not been well studied. It will be necessary to examine these species in order to determine the phylogenetic position of D. pectinifera among the montium species subgroup in Asia.
We thank Drs. T. A. Markow and J. Bono for critical reading of the manuscript; E. T. Matsuura and two anonymous reviewers for critical comments on the manuscript; Dr. M. T. Kimura for information about the auraria complex; and Masashi Matsumoto for technical assistance. We also thank Dr. Y. Fuyama (Tokyo Metropolitan University) and Dr. Y. Oguma (University of Tsukuba) for fly stocks. Most of the fly stocks used in this study were provided by the National BioResource Project (NBRP) of Japan.
|