| Edited by Fujio Kawamura. Hisaji Maki: Corresponding author. E-mail: maki@bs.naist.jp. Footnotes: Satoshi Kanie, Katsuyoshi Horibata and Mitsuoki Kawano. These three persons were equally contributed to this work. Abbreviations: Amp, ampicilin; Can, chloramphenycol, Kan, kanamycin; Str, streptomycin |
We have previously proposed a hypothesis that the majority of spontaneous mutations are not derived from replication errors but caused by other premutagenic DNA lesions that are never or inefficiently corrected by the mismatch repair system (Fujii et al., 1999; Yoshiyama et al., 2001; Yoshiyama and Maki 2003). This is based on our findings that patterns of spontaneous mutations occurring in a wild-type Escherichia coli strain were dissimilar to those in a mismatch-repair (MMR) deficient strain, in which replication errors are not corrected and turn into the mutations. Using the rpsL mutation assay, by which forward mutations within rpsL target sequence placed on a multi-copy plasmid can be easily selected, a large number of spontaneous mutations recovered from wild-type and MMR–strains were examined in their mutational classes and locations within the target sequence. Unique characteristics for the mutations caused by replication errors were that the most frequent type of mutation was single-base frameshifts occurring exclusively at runs of the same nucleotides and that base substitutions were randomly distributed throughout the target sequence. These characteristics were not seen in spontaneous mutations recovered from the wild-type cells. Instead, non-random distributions in the class and the location of single-base mutations and base substitutions were characteristics for the spontaneous mutations in wild-type cells. The rpsL target sequence appeared to contain various hotspot sites for both types of point mutations occurring in the wild-type strain, while frequencies of mutations at these hotspots were unchanged in the MMR–strain. In the reverse, the hotspot mutations occurring in the MMR–strain became infrequent mutations in the wild-type strain. These imply that the vast majority of replication errors are highly efficiently corrected by the MMR and that the hotspot mutations occurring in the wild-type strain are those not subjected to the MMR. Since trace of the signature mutations for MMR–strain was feeble in non-hotspot mutations occurring in the wild-type cells, the significance of replication errors for the spontaneous mutations seems to be marginal unless cells lose the vital function of MMR. Therefore, an important question is what are the premutagenic lesions that cause the hotspot-type as well as the non-hotspot type of spontaneous point mutations (Maki 2002).
During the study of spontaneous rpsL mutations, we obtained a hint on the hotspot mutagenesis. A significant portion of hotspot-type frameshifts and base substitutions appeared to be produced in a manner similar to one generating sequence substitution mutations, which involve the conversion of pseudo-inverted repeats into perfectly inverted repeats (Maki 2002; Mo et al., 1991; Yoshiyama et al., 2001). These sequence substitutions and the related hotspot-type mutations were significantly affected by a mutator mutation in dnaE gene that encodes a catalytic subunit of DNA polymerase III (Mo et al., 1991), directionality of DNA replication fork movement (Yoshiyama et al., 2001), and the level of transcription (Yoshiyama and Maki 2003). It should be emphasized that most of these mutations are resistant to the MMR. However, since the MMR system efficiently suppresses sequence substitutions of one or two nucleotides (T. Muroya, T. Ohsu, and HM, unpublished results), the hotspot point mutations that are totally resistant to mismatch correction seem to be something other than just a small sequence substitution. Probably, such premutagenic lesions are produced when the DNA replication fork encounters spontaneous DNA lesions, such as oxidatively damaged base and abasic lesion. DNA synthesis against such a lesion on the template by replicative or translesion DNA polymerases would result in a mismatched site with the lesion, which is hardly corrected by the mismatch repair system.
To further verify the hypothesis, we have started to examine involvement of DNA transactions that process various kinds of DNA lesions in the spontaneous mutagenesis. It has been widely accepted that DNA lesions and the resulting stall of replication fork are processed by three pathways of DNA transactions; recombinational repair, replication fork regression, and translesion DNA synthesis (Cox 2001). The former two pathways that require functions of RecA protein are essentially error-free, while the translesion DNA synthesis are relatively error-prone. To evaluate anti-mutagenic and mutagenic actions of these DNA transactions at the same time, we have developed a new rpsL system, by which recombination events as well as forward mutations occurring within about 600 base pairs of a target sequence inserted into Escherichia coli chromosome can be examined. Using the chromosomal rpsL system, we extensively analyzed spontaneous mutations and allelic recombination events in a wild-type Escherichia coli strain and its derivative defective in the RecA function. In a wild-type strain, the recombination events were occurring with a frequency comparable to that of spontaneous point mutations including base substitutions and single-base frameshifts. A recA null mutation led to a sharp decline of the recombination events and significantly more frequent occurrences of single-base frameshifts and large deletions. In the present article, we describe a novel mutator phenotype of the recA mutant strain.
The bacterial strains used in this study were all derivatives of E. coli K12 and shown in Table 1. MG1655, ES1301, and DB1318 were obtained from the E. coli Genetic Stock Center, Yale University. Strains MK811 (wild type), MK1017 (ΔrecA), MK1125 (ΔmutS), and MK1137 (ΔrecA ΔmutS), in which a 735 bp DNA fragment containing the wild-type rpsL gene was inserted at cysJIH locus on E. coli chromosome, were used as tester strains for chromosome-based rpsL mutation assay. MK700 and MK801 were Strr derivatives of MK426 and MG1655, respectively, and obtained by plating the cells onto LB containing streptomycin. The responsible mutation (rpsL128) in rpsL gene was determined for both strains by DNA sequencing. recA-null mutant strains MK427, MK1017, and MK1137 were constructed from MK426, MK811, and MK1125, respectively, by P1-mediated transduction with DB1317 as a donor followed by Camr selection. MK1125 was constructed from MK811 by P1-mediated transduction with ES1301 as a donor followed by Kanr selection. LB contained 1% (w/v) Bactotryptone (Difco), 0.5% (w/v) yeast extract (Difco), and 1% (w/v) NaCl. LB plates were solidified with 1.5% Bacto agar (Difco). Ampicillin, chloramphenicol, and streptomycin were added to the medium when needed at concentrations of 100 μg/ml. Kanamycin was added to the medium when needed at 40 μg/ml.
 View Details | Table 1. Bacterial strains |
In order to construct a rpsL hemi-diploid strain, about 2.6 kb DNA segment containing bla (Ampr marker) gene, a transcription terminator sequence rrnBT1T2, and rpsL gene was inserted into a promoter region of cysJIH operon, located at 59 min on E. coli genome (Fig. 1). This insertion resulted in a deletion of 802 bp of upstream region of this operon, which covers the promoter/operator region and a 5’-terminal portion of the cysJ gene. Therefore, transcription of the rpsL transgene would not be affected by the surrounding sequence but governed solely by its own promoter. The left arm (KpnI-NotI) and the right arm (NotI-SacI) attached to the 2.6 kb DNA segment were obtained by PCR with primer sets, dKPNICR-dNOTICF and dNOTICR-dSACICF, respectively, and MK426 genome DNA as template and ligated to SacI-KpnI digested pSTV28 DNA (TAKARA BIO Inc.), resulting in plasmid pKurabe1. The 2.6 kb DNA segment was obtained from plasmid pCE1 and gel-purified after NotI digestion of this plasmid. The pCE1 was derived from pMOL21 (Yoshiyama et al., 2001) by introducing two NotI recognition sequences at sites 1202 and 2797 and by replacing rpsL(am) with a 740 bp BamHI-NotI fragment carrying rpsL+ that was amplified by PCR using a primer set, dBAMHI21R-dNOTIEGF, and MK426 genome DNA as template. The 2.6 kb NotI fragment was ligated into a unique NotI site of pKurabe1, resulting in plasmid pCE2. 6.0 kb SacI-KpnI fragment DNA was prepared from pCE2 and introduced into MK700 cells by electroporation, and Ampr recombinant clones were selected. Two out of 21 Ampr clones showed Cys–Strs phenotype, and the location and directionality of rpsL transgene was confirmed by PCR. One of the clone, MK701, was chosen as a donor strain for a further P1-transduction to construct MK811, in which the P1-phage infected MK801 cells were screened for Ampr.
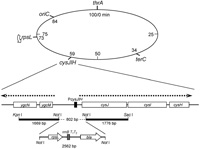 View Details | Fig. 1. Integration of rpsL transgene into E. coli chromosome at cysJIH locus. The E. coli linkage map is shown in the upper part. Genome structure around the cysJIH operon is illustrated under the linkage map. Open boxes indicate coding sequences, and PcysJIH is a promoter/operator segment for the operon. Directions of transcription for cysJIH operon, ygcM and ygcN are indicated by arrows above the genes. DNA segments used for the homologous-recombination driven gene targeting are shown by thick lines with restriction enzyme recognition sites. 2562 bp DNA fragment carrying rpsL and bla genes is also indicated. |
The plasmid-based rpsL mutation assay was carried out with a tester plasmid pMOL21 as described (Yoshiyama et al., 2001). The chromosome-based rpsL mutation assay was performed with MK811 and its derivatives. Cells were fully grown in LB at 30°C, and approximately 100 cells were inoculated into a test tube containing 5 ml LB. The cells were grown at 37°C with agitation (160 rpm using Roller drums model TC-7, New Brunswick Scientific) until OD600 = 1.0, and appropriate dilutions were spread on LB plates to determine the number of total cells and on LB plates containing streptomycin to determine rpsL–cells. Mutation frequency for each cell culture was calculated by dividing the number of mutant cells by the total number of cells. Average frequencies of total mutations and each class of mutations were calculated as described (Fujii et al., 1999), except that the jackpot mutations were excluded from the calculation.
All primers used in this study were supplied by Greiner Japan and are listed in Table 2. PCR procedures were as described (Hiraoka, et al., 2000). The rpsL coding region and its surrounding sequence (from position –130 to position 385) in each mutant cell were determined using an automated DNA sequencer (Megabase 1000, Amersham Pharmacia Biotech).
 View Details | Table 2. Primers for PCR |
MK811 cells were grown at 37°C in LB until OD600 = 0.2, precipitated by centrifuge at 4°C, resuspended in a half volume of 0.9% NaCl solution, and irradiated with UV (300 J/m2). 5 ml of LB was added to 1 ml of the UV-irradiated cell suspension, and the cells were grown at 37°C for 8 h before determination of rpsL– mutation frequency.
We have previously analyzed forward mutations using the plasmid-based rpsL system that requires extraction of propagated plasmid DNA from a host strain and transformation of a tester strain with the purified plasmid DNA when rpsL mutant plasmids are selected (Fig. 2a). To establish a versatile assay for forward mutations occurring in the same target gene placed within chromosome, we constructed an E. coli strain, MK811, in which 735-bp of rpsL fragment was inserted at a particular site within cysJIH operon located at 59 min in the linkage map of E. coli chromosome (Fig. 1). In MK811, the authentic rpsL gene located at 73 min is a Strr allele (rpsL128) resulting from an A:T → C:G transversion at site 128 in the gene, while the transgenic rpsL gene located at 59 min is a wild-type allele. The hemi-diploid rpsL strain gains a Strs phenotype because of the recessive nature of Strr allele. Thus, genetic alterations that hamper the function of transgenic rpsL gene would revert the phenotype back to Strr (Fig. 2b). With a new assay protocol using the chromosome-based rpsL system, rpsL mutants can be screened simply by plating the hemi-diploid cells onto LB media containing streptomycin.
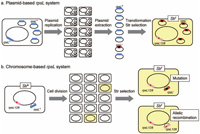 View Details | Fig. 2. Schematic illustration of plasmid-based and chromosome-based rpsL mutation assay systems. |
As summarized in Table 3, frequencies of base substitution and single-base frameshift detected by the chromosome-based rpsL system in the wild-type strain (MK811) were very close to those determined with the plasmid-based system. From further analysis of 567 samples of base substitution recovered from the chromosome-based system, it appeared that about half of the base substitutions were occurring at several hot-spot sites (Fig. 3). This non-random site-distribution of base substitutions was also observed in the plasmid-based system (Yoshiyama et al., 2001). Surprisingly, the site-distribution of these hot-spot mutations in chromosome-based and plasmid-based systems were different. In particular, we found two strong hot-spot type of mutations unique to the chromosome-based system, T:A → A:T transversion at site 245 and C:G → A:T transversion at site 82, which accounted for 38% and 8.8% of base substitutions occurring within the chromosomal rpsL target sequence, respectively. Neither 245T→A nor 82C→A was detected among 120 base substitution mutations analyzed with the plasmid-based system, whereas hot-spot type of base substitutions in the plasmid-based system were 3G→A, 73G→T, 101G→A, 107G→C, 184G→T, 208G→T, and 285C→A. In the spectra of 90 single-base frameshifts from chromosome-based system (Fig. 4), single-base deletions were predominant and distributed randomly throughout the target sequence. There was a mild hot-spot for single-base addition at a run of 6 residues of dA. These contrast with the site-distributions of single-base frameshifts in the plasmid-based rpsL system, to which single-base additions at non-iterative sites were peculiar (Yoshiyama et al., 2001). Duplication mutations and insertion events of IS elements were also detected in both chromosome-and plasmid-based systems at similar levels of the frequencies. On the other hand, clear differences between the two rpsL systems were seen in occurrences of sequence substitution and large deletion (Table 3). In the chromosome-based system, frequency of sequence substitution was below the detection limit, 7.0 × 10–10, at least 240-fold lower than that detected in the plasmid-based system. Large deletion was 23-fold less frequent in the chromosome-based system when compared with the plasmid-based system.
 View Details | Table 3. Class distributions of rpsL forward mutations detected by plasmid-based and chromosome-based systems in various E. coli strains |
 View Details | Fig. 3. Site distribution of base substitutions identified in rpsL–clones derived from a wild-type E. coli strain, MK811. Sequence changes found in mutants of MK811 are shown in colors above or below the rpsL sequence. Nucleotide positions starting with the first position of the initiation codon are shown on the right side of the sequence. The promoter region (–35 and –10), the Shine-Dalgano sequence, and the initiation and termination codons are underlined. Mutations identified in the same cell culture are shown in the same color. Numbers added in the right side of altered bases are numbers identified in the same cell culture. |
 View Details | Fig. 4. Site distribution of single-base frameshifts identified in rpsL–clones derived from a wild-type E. coli strain, MK811. Sequence changes found in mutants of MK811 are shown in colors above or below the rpsL sequence. Nucleotide positions and other elements of DNA sequence are indicated as in Fig. 3. Single-base deletions are indicated by∇, and single-base additions are shown by nucleotides above∨. Mutations identified in the same cell culture are shown in the same color. |
From these data, it was revealed, for the first time, that the nature of spontaneous mutagenesis on chromosome DNA is distinct from that on plasmid DNA especially in the mutagenesis for hot-spot type base substitutions, sequence substitutions, and large deletions. However, non-hot-spot type mutations of base substitution and single-base frameshift, duplication mutations, and insertions of IS elements seem to occur in a way common to both chromosome and plasmid.
The hemi-diploid situation for rpsL gene in the chromosome-based rpsL system would provide a chance to detect spontaneous recombination events, namely non-reciprocal allelic recombination between the transgenic rpsL+ allele and the authentic rpsL128 allele (Fig. 2). As shown in Table 3, this actually happened in the wild-type strain, MK811. 22% of Strs → Strr mutations recovered from this strain were A:T → C:G transversions at site 128 (128A→C) in the rpsL sequence, exactly the same mutation as rpsL128 allele.
Several lines of evidence support the notion that the 128A→C, the most frequent single type of genetic alterations in MK811, is due to allelic recombination between the homologous rpsL genes in the strain. First of all, when we isolated rpsL128 allele from a wild type strain MG1655 with a monoploid rpsL+ gene, the 128A→C appeared to be a rare mutation with a frequency around the order of 10–10, 1000-fold lower than the case of the same alteration in MK811. Therefore, the extremely high frequency of 128A→C is dependent on the hemi-diploid situation of rpsL gene. Secondly, about 70% of the 128A→C recovered from MK811 accompanied with another base change, G:C → A:T transition at site –22, where a polymorphism (G for the transgenic rpsL versus A for the authentic rpsL) exists. The simultaneous base-change at sites 128 and –22 can only be explained by recombination events with a patch size longer than 140 bp. Thirdly, the frequency of 128A→C was sharply decreased to an undetectable level when recA gene was deleted (Table 3, MK1017). Finally, in reverse, the frequency of 128Α→C was increased about 34-fold when the mutS-dependent mismatch repair system was hampered (Table 3, MK1381). Since the frequency of 128A→C in a mutS–recA–double mutant strain, MK1137, was as low as the recA–strain, the increased level of 128A→C in the mutS–strain was not due to uncorrected replication errors. It has been known that the mismatch repair system has an anti-recombination function called heteroduplex rejection, which destroys recombination-intermediates containing mispairs (Schofield and Hsieh 2003). This function probably eliminates mispairs formed in heteroduplex between rpsL128 and rpsL+ and suppresses 128A→C. Considering the above findings together, we concluded that the chromosome-based rpsL system provides a sensitive and useful assay for not only forward mutations but also chromosomal recombination events.
The allelic recombination detected in MK811 must require cellular events initiating homologous DNA recombination, which likely involves double-strand DNA breaks and/or inhibition of DNA chain elongation. In fact, the allelic recombination was readily induced in MK811 after the cells UV-irradiated (Table 4) or treated with H2O2 (data not shown). Therefore, it seemed conceivable that spontaneous DNA lesions which initiate the allelic recombination are constitutively produced in MK811 cells grown under the normal condition.
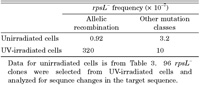 View Details | Table 4. Induction of allelic recombination by UV-irradiation to MK811 cells |
The spontaneous recombination events detectable in the chromosome-based rpsL system were essentially vanished in the ΔrecA strain, implying that a significant amount of spontaneous DNA lesions are processed by cellular functions requiring the RecA protein. If such DNA lesions are not processed by the RecA functions, cells would become genetically more unstable. This appeared to be the case. The ΔrecA strain showed a moderate mutator phenotype in both chromosome- and plasmid-based rpsL systems (Table 3). Although the mutator effect was marginal on base-substitution mutagenesis, frequencies of single-base frameshift and large deletion in the ΔrecA strain were 5- to 10-fold higher than the wild-type levels. 10-fold increasement of duplication mutations by the recA mutator effect was also observed with the plasmid-based system. As shown in Table 3, frequency of single-base frameshift in MK1137 (mutS–recA–) strain was 400-fold and 8-fold higher than those in MK1017 (mutS+ recA–) and MK1381 (mutS–recA+), respectively. Therefore, single-base frameshifts induced in the ΔrecA background are highly effectively suppressed by the mutS-dependent mismatch repair system, and cells lacking RecA protein produce replication errors leading to single-base frameshifts 8-fold more frequently than in the recA-proficient cells.
These data clearly indicate that the RecA protein plays an important role in maintaining the spontaneous mutations at a certain low level by properly processing the spontaneous DNA lesions. Furthermore, it becomes now evident that spontaneous DNA lesions are a potential source for single-base frameshift, large deletion and duplication mutagenesis in Escherichia coli.
Genetic alterations in organisms involve point mutations as well as recombination events leading to various kinds of chromosomal rearrangements. We have proven the chromosome-based rpsL mutation assay system to be useful for analyzing at the same time both point mutations and recombination events occurring within the same target sequence. Interestingly, spontaneous mutations occurring in the chromosomal rpsL transgene were distinct from those detected with the plasmid-based rpsL mutation assay. In particular, no sequence substitution was detected in the chromosomal rpsL mutations, while 11% of the plasmid-born rpsL mutations were sequence substitutions (Yoshiyama et al., 2001). Most recently, we found that E. coli cells possess an avoidance-mechanism specific for sequence substitution mutagenesis and that mutant strains defective in the mutation-avoidance mechanism show elevated frequencies of sequence substitution even in the chromosomal rpsL target sequence (Yoshiyama and Maki, unpublished results). Since most of the hot-spot type of point mutations in plasmid-born rpsL are likely caused by sequence-substitution mutagenesis (Yoshiyama et al., 2001), such hot-spot mutations could be suppressed in the chromosomal rpsL target sequence by the same reason as the sequence substitutions are suppressed. Recently, we showed that oxidative DNA lesions are involved in mutagenesis for the hot-spot type of base substitutions in the chromosomal rpsL target sequence (Sakai et al., 2006). The reason why the chromosomal hot-spot mutations were not found in the plasmid-born rpsL mutations is unknown.
Using the chromosome-based rpsL mutation assay, we revealed that allelic recombination events occur almost as frequently as point mutations in a normally growing wild-type E. coli cells. In the plasmid-based rpsL system, such allelic recombination events may occur, but from a multiple copy number of the plasmid most of the events would be those between plasmid-born rpsL+ segments rather than those with the chromosomal rpsL128 allele. This is probably the reason why the allelic recombination events are not found in the plasmid-based rpsL mutation assay. It should be noted that the allelic recombination detected in the rpsL system is a gene-conversion type of homologous recombination between 740 bp of DNA segments about 640 kb apart from each other in the E. coli genome. It has been proposed that replication-fork blockages are readily recovered by homologous recombination between sister chromatids in the vicinity of the blockage (Cox 2001). However, in some instance unsuccessful sister-chromatid exchange would result in the allelic recombination. If this is the case, spontaneous recombination events, mostly sister-chromatid exchange, occurring within the target sequence should be much more frequent than those for the allelic recombination in the normally growing wild-type cells. This notion is consistent with a highly frequent generation of spontaneous DNA lesion in aerobically growing E. coli cells (Sakai et al., 2006).
In the present study, we found a novel mutator phenotype of a ΔrecA strain. In the recA-deficient cells, frequencies of single-base frameshifts and large deletions occurring in the chromosomal rpsL target sequence were 5-fold and 8-fold higher than those determined with a co-isogenic recA-proficient strain. It has been well documented that the RecA protein participates in major pathways maintaining genome stability threatened by replication blocking DNA damages (Cox 2001). Furthermore, besides direct roles in the repair processes for stalled DNA replication fork, the RecA protein acts as a key factor that initiates and regulates the SOS response, a cellular response to DNA damage in bacterial cells (Cox 2001). In normally growing E. coli cells, a sub-population of the cells would suffer from the replication-fork blockage caused by spontaneous DNA lesion, and such cells may be expressing the SOS response to recover the blockage and to maintain the genome integrity. Therefore, the mutator phenotype of recA-null mutant may be due to an improper processing of the replication-fork blockage.
When homologous recombination pathway is hampered, non-homologous recombination pathway may substitute for the major pathway repairing double strand breaks. An end-joining type of recombination may be responsible for the increased frequency of large deletions in the recA-null mutant. On the other hand, the elevated occurrence of single-base frameshift could be resulted from an error-prone DNA synthesis by translesion DNA polymerases. Among the E. coli translesion DNA polymerases, DNA polymerases IV (DinB) and V (UmuCD’2) are devoid of the proofreading exonuclease activity and a very poor fidelity of DNA synthesis. In addition, unlike Pol V, Pol IV is present abundantly in normally growing cells and does not require the RecA protein for the translesion as well as usual DNA synthesis. Furthermore, a significantly increased frequency of single-base frameshift was observed when the Pol IV was overproduced in E. coli cells (Wagner and Nohmi 2000). Therefore, it seems probable that the Pol IV might be responsible for the increased level of single-base frameshift in the recA-null mutant. However, the single-base frameshift induced in the recA mutant is not simply caused by spontaneous DNA lesions but likely due to an elevated incidence of replication error. This is because the ΔrecA-dependent single-base frameshifts are strongly suppressed by mutS-dependent mismatch repair. In the ΔrecA mutant cells, slippage errors were made during DNA replication 8-times more frequently than in the recA-proficient cells. Probably, most of the slippage errors occur during an error-prone DNA synthesis on undamaged template DNA. At present, we have no persuasive explanation for such an error-prone DNA synthesis in the recA-null mutant. An attractive hypothesis is that the RecA protein might protect chromosomal DNA replication from an unnecessary action of Pol IV. Although we showed an important role of RecA protein in suppressing spontaneous mutations, at least single-base frameshifts and large deletions, we have to await further studies on molecular mechanisms for the RecA-mediated mutation avoidance process.
We thank Keiko Ida for DNA sequencing analyses. We acknowledge the financial support of Grants-in-Aid for Scientific Research on Priority Areas (12213082 to H. M.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. A. S. was supported by the 21st Century Center of Excellence Program (Graduate School of Biological Sciences, NAIST) from the Japan Society for the Promotion of Science.
|