| Edited by Takashi Endo. Takeshi Nishio: Corresponding author. E-mail: nishio@bios.tohoku.ac.jp |
Retrotransposons are ubiquitous in the plant kingdom (Kumar and Bennetzen, 1999), but those still having transposing activity, such as Tos17, Tto1, Tnt1, and LORE1 (Grandbastien et al., 1989; Hirochika, 1993; Hirochika et al., 1996; Madsen et al., 2005), are few in plants. Retrotransposons can be divided into two groups, i.e., LTR (long terminal repeat) retrotransposons and non-LTR retrotransposons, and the LTR retrotransposons are classified into Ty1-copia and Ty3-gypsy (Xiong and Eickbush, 1990). The copy number of retrotransposons tends to increase because of their copy-and-paste mode of transposition. Retrotransposons have been estimated to constitute approximately 80% of the genome in maize, which has a large genome size (SanMiguel et al., 1998), but less than 10% in Arabidopsis thaliana, which has a small genome size (Arabidopsis Genome Initiative, 2000). Transposition and amplification of retrotransposons are considered to play a major role in the evolution of plant genomes.
Brassicaceae consists of about 340 genera and more than 3,350 species including important crops and a model plant, A. thaliana (Al-Shehbaz, 1984). Important crop species, such as Brassica oleracea, Brassica rapa, Brassica napus, and Raphanus sativus, belong to the tribe Brassiceae. Brassica juncea, Brassica carinata, and B. napus are allopolyploids having AABB, BBCC, and AACC genomes, respectively, the A, B, and C genomes of which are derived from B. rapa, Brassica nigra, and B. oleracea, respectively (U, 1935). Brassicaceae is an interesting family for the study of genome evolution because of its large variations of chromosome number and genome size (Arumuganathan and Earle, 1991; Johnston et al., 2005).
Many species in Brassicaceae have self-incompatibility, which is controlled by the S locus (Bateman, 1955). About 50 and 30 S haplotypes have been identified in B. oleracea and B. rapa, respectively (Nou et al., 1993; Ockendon, 2000). Interspecific pairs of S haplotypes having the same recognition specificity have been identified between B. rapa and B. oleracea (Kimura et al., 2002; Sato et al., 2003). Comparison of the genome structure of the S locus region between interspecific pairs has revealed that the S locus in B. oleracea is larger than that in B. rapa, the differences of the S locus sizes between them being due to insertion of retrotransposons. The retrotransposons identified in the S-locus region have been named STF (S locus retrotransposon family). Southern blot analyses using STF sequences as probes have revealed that B. rapa and B. oleracea have some STF homologs in common but that other STF homologs are present only in one species. More signals of STF homologs have been detected in B. oleracea than in B. rapa (Fujimoto et al., 2006a).
In the present study, we sequenced many homologs of five STFs, i.e., BoSTF7a, BoSTF12a/15a, BoSTF12b, and BrSTF60a, classified into a Ty3-gypsy group (Fujimoto et al., 2006a), and BrSTFf2a, classified into a Ty1-copia group (Fujimoto et al., 2006b). We also sequenced and phylogenetically analyzed Tto1 in Brassica, classified into the Ty1-copia group.
F1 hybrid cultivars of Chinese cabbage, ‘CR-Seiga 65’ (Ishii Seed Co.) and ‘Cream No. 2’ (Watanabe Seed Co.); komatsuna, ‘Osome’ (Takii Seed Co.); pak-choi, ‘Seibu’ (Sakata Seed Co.); turnip, ‘Wase-Ohkabu’ (Takii Seed Co.); and oil seed rape, ‘Yellow Sarson’ (C634; Tohoku University Brassica Seed Bank, http://www.agri.tohoku.ac.jp/pbreed/Seed_Stock_DB/SeedStock-top.html) were used as materials of Brassica rapa. F1 hybrid cultivars of broccoli, ‘Greencomet’ (Takii Seed Co.) and ‘Ryokurei’ (Sakata Seed Co.), cabbage, ‘CM’ (Takii Seed Co.), and cauliflower, ‘Bridal’ (Sakata Seed Co.), and inbred lines of kale and Chinese kale were used as materials of Brassica oleracea. Genomic DNA for Southern blot analysis was isolated from leaves by the CTAB method (Murray and Thompson, 1980).
Ten species in Brassicaceae, i.e., B. carinata (CA-114), B. juncea (J-117), B. napus (‘Westar’), B. nigra (Ni-140), B. tournefortii (T-162), Diplotaxis erucoides (DIP-ERU-9), Eruca sativa (ERU-SAT-1), Raphanus sativus (RAP-SAT-29), Sinapis alba (SIN-ALB-25), and Sinapis arvensis (SIN-ARV-13), which are maintained in the Tohoku University Brassica Seed Bank, were used for analysis of retrotransposon sequences. Genomic DNA was isolated from single seeds according to Sakamoto et al. (2000). Retrotransposons were amplified by PCR using the primers listed in Table 1. Amplified DNA was cloned using pGEM-T Easy Vector System I (Promega, WI, USA).
 View Details | Table 1 Sequences of primers used for PCR amplifications |
Genomic DNA (2 μg) digested with EcoRI was electrophoresed on 1.0% agarose gel and transferred onto a nylon membrane (Nytran, Whatman, UK). The membrane was hybridized with a digoxigenin-labeled probe at 65°C. The rvt region of BrSTF60a, the rnaseH region of BrTto1/BoTto1, and the gag region of BoSTF7a, BoSTF12a/15a, BoSTF12b, and BrSTFf2a were used as probes. The gag region was mainly used as a probe because of its specificity. After hybridization, the membrane was washed twice in a solution of 0.1% SSC containing 0.1% SDS at 65°C for 20 min, and signals were detected according to an instruction manual (Roche, Rotkreuz, Switzerland).
Nucleotide sequences of 24 clones of PCR products were determined with a CEQ 2000XL DNA Analyzer (Beckman Coulter, CA, USA), and the data were analyzed using Sequencher (Gene Codes Corporation, MI, USA). The sequences were aligned using ClustalW (http://www.ddbj.nig.ac.jp/search/clustalw-j.html). The average number of substitution was calculated by PAUP ver. 4.0 (Swofford, 1998). Phylogenetic trees were constructed with the neighbor joining method (Saitou and Nei, 1987), and bootstrap probabilities of 1,000 trials were calculated.
The pattern of divergence of the retrotransposons was investigated using Brassicaceae species. The six putative retrotransposons (i.e., putative homologs of five STFs and one Tto1) were isolated from B. rapa and B. oleracea genomic DNAs by PCR with the same primer sets and reaction cycle conditions. To reveal the copy number of STFs and Tto1 in the Brassica genome, Southern blot analyses were performed using genomic DNAs of six cultivars each in B. rapa and B. oleracea. The number of bands detected by Southern blot analyses with six retrotransposon probes was evaluated using the following scores: 0, no band; 1, one band; 2, 2–5 bands; 3, more than 6 bands; and 4, smear (Fig. 1). The results for B. rapa and B. oleracea are summarized in Table 2. The copy number of retrotransposons in B. oleracea was generally larger than that in B. rapa. The average scores of all six retrotransposons were 3.3 and 2.5 in B. oleracea and B. rapa, respectively.
 View Details | Fig. 1 Southern blot analysis of genomic DNAs of six cultivars in B. rapa and B. oleracea. After electrophoresis, genomic DNA digested with Eco RI was hybridized with the probes of the gag regions of BoSTF12a and BoSTF12b and the rnaseH region of Tto1. Lanes 1 to 6 in B. rapa are as follows: 1. ‘Osome’; 2. ‘CR-Seiga 65’; 3. ‘Cream No. 2’; 4. ‘Seibu’; 5. ‘Wase-Ohkabu’; 6. ‘Yellow Sarson’. Lanes 1 to 6 in B. oleracea are as follows: 1. ‘Greencomet’; 2. ‘Ryokurei’; 3. ‘CM’; 4. ‘Bridal’; 5. Chinese kale; 6. Kale. The scores estimated by Southern blot analyses are shown in Table 2. |
 View Details | Table 2 Scores of band numbers detected by Southern blot analyses using retrotransposon probes |
Twenty-four randomly selected clones of PCR products for the six retrotransposons, which were amplified from genomic DNAs of B. rapa cv. ‘Osome’ and B. oleracea cv. ‘Greencomet’, were sequenced to reveal their divergence patterns in the genome. For each retrotransposon, phylogenetic relationships were inferred by the neighbor-joining method (Fig. 2). Species-specific clades of B. rapa and B. oleracea were observed in the phylogenetic trees of BrSTF60a-rvt and BrSTFf2a-gag. Tto1-rnaseH also showed species-specific clades except for one B. rapa sequence in the B. oleracea clade. The remaining sequences showed trans-specific patterns, indicating the retention of ancestral sequences of retrotransposons in various genomic regions.
 View Details | Fig. 2 Neighbor-joining trees of nucleotide sequences of the gag and rvt regions of STFs and the rnaseH region of Tto1 in B. rapa and B. oleracea. Black and gray lines indicate the sequences of B. oleracea and B. rapa, respectively. Bo, B. oleracea; Br, B. rapa. |
The branch lengths in B. oleracea clades seem longer than those in B. rapa, implying higher diversity of the retrotransposons in B. oleracea (Fig. 2). Indeed, the average number of substitutions in pairwise comparison between the 24 retrotransposons revealed significantly more nucleotide substitutions in five of the six retrotransposons in B. oleracea than in B. rapa (P < 0.01; Table 3). The results of the average number of substitutions may reflect the difference of retrotransposon copy number between B. oleracea and B. rapa.
 View Details | Table 3 Average number of substitutions of six retrotransposons in B. rapa and B. oleracea |
Since higher copy number and diversity of retrotransposons were found in B. oleracea than in B. rapa, whose genome size is about 100-Mb smaller than that of B. oleracea (Arumuganathan and Earle, 1991), we investigated the relationship between genome size and the number of substitutions in two retrotransposons using nine diploids of Brassicaceae. Twenty-four clones of homologs of BoSTF7a and Tto1 amplified from the genomic DNAs of nine diploids by PCR were sequenced. The estimated average number of substitutions and the genome size according to Arumuganathan and Earle (1991) are shown in Table 4. A significant positive correlation between genome size and the average number of substitutions of the BoSTF7a homologs in the nine species was observed (r = 0.726, P = 0.0261, permutation test) (Table 4, Fig. 3A), but genome size showed no correlation with the average number of substitutions in the Tto1 homolog (r = 0.0465, P = 0.413, permutation test) (Table 4, Fig. 3B).
 View Details | Table 4 Average number of substitutions of BoSTF7a homolog and Tto1 homolog in Brassicaceae species |
 View Details | Fig. 3 Correlation between genome size and average number of substitutions in STF7a (A) and Tto1 (B) (shown in Table 4) and between genome size and number of non-functional STF7a (C) and Tto1 (D) (shown in Table 5) in nine diploids of Brassicaceae. The significance of the coefficient was computed by permutation test with 10,000 randomizations. Bni, B. nigra; Bo, B. oleracea; Br, B. rapa; Bt, B. tournefortii; De, D. erucoides; Es, E. sativa; Sal, S. alba; Sar, S. arvensis; Rs, R. sativus. |
Based on cDNA sequences, the numbers of nonsense mutations and frameshift mutations in the 24 clones of the BoSTF7a homologs and the Tto1 homologs were investigated in nine diploid species. There were more nonsense mutations than frameshift mutations in the BoSTF7a homologs. There were also more nonsense mutations and frameshift mutations by deletion than frameshift mutations by insertion in the Tto1 homologs (Table 5). The number of deletions in the BoSTF7a sequences showed significant correlation with the genome size in nine diploids, but there was no correlation between the number of insertions in the BoSTF7a sequences and the genome size (Table 6). The numbers of deletions and insertions in Tto1 showed no correlation with the genome size, either. Positive correlations between genome size and deletion size in the BoSTF7a sequences and between genome size and insertion size in the Tto1 sequences were observed (Table 6). Genome size showed a correlation with the number of non-functional BoSTF7a sequences caused by nonsense mutations or frameshift mutations in the nine species (r = 0.733, P = 0.0116, permutation test) (Table 5, Fig. 3C), but showed no correlation with the number of non-functional Tto1 homologs in those species (r = 0.241, P = 0.272, permutation test) (Table 5, Fig. 3D).
 View Details | Table 5 Number of nonsense mutations and frameshift mutations for insertion and deletion of BoSTF7a homolog and Tto1 homolog in Brassicaceae species |
 View Details | Table 6 Correlation coefficient between genome size and frequency/size of insertion and deletion in STF7a and Tto1 homologs in Brassicaceae species |
The divergence pattern of retrotransposons can provide further insight into the contribution of genome size evolution. Phylogenetic trees of nucleotide sequences of the BoSTF7a and Tto1 homologs in nine diploids and three allopolyploids in Brassicaceae were inferred. Both the BoSTF7a and Tto1 homolog sequences of eight diploid species clustered in one to three species-specific clades of retrotransposons (Fig. 4, Fig. 5). Multiple clades in each diploid species suggest that some types of retrotransposons may have been amplified and that others may have been lost after speciation. On the other hand, retrotransposons of S. alba and allopolyploids showed different patterns (Fig. 4, Fig. 5). The BoSTF7a homolog sequences, but not Tto1, of S. alba were clustered into clades of B. rapa and B. oleracea. The BoSTF7a and Tto1 homolog sequences of allopolyploids were clustered into clades of their original genomes, e.g., the sequences of B. juncea (AABB genome) belonging to the clade of B. rapa (AA genome) and that of B. nigra (BB genome).
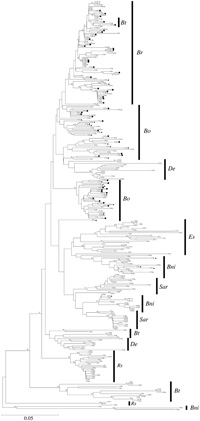 View Details | Fig. 4 A neighbor-joining tree of nucleotide sequences of the gag region of STF7a. Bootstrap values with 1,000 replicates are indicated at the node of the neighbor-joining trees. Bold crosses, filled triangles, squares, and circles represent the clones of allotetraploids species, B. juncea, B. carinata, B. napus, and diploid species S. alba, respectively. Bni, B. nigra; Bo, B. oleracea; Br, B. rapa; Bt, B. tournefortii; De, D. erucoides; Es, E. sativa; Sar, S. arvensis; Rs, R. sativus. |
 View Details | Fig. 5 A neighbor-joining tree of nucleotide sequences of the rnaseH region of Tto1. Bootstrap values with 1,000 replicates are indicated at the node of the neighbor-joining trees. Bold crosses, filled triangles, and squares represent the clones of allotetraploids species, B. juncea, B. carinata, and B. napus, respectively. Bni, B. nigra; Bo, B. oleracea; Br, B. rapa; Bt, B. tournefortii; De, D. erucoides; Es, E. sativa; Sal, S. alba; Sar, S. arvensis; Rs, R. sativus. |
More bands were generally detected in B. oleracea than in B. rapa by Southern blot analysis, indicating the presence of more retrotransposons in B. oleracea than in B. rapa, as was suggested in our previous study (Fujimoto et al., 2006a). The average number of substitutions of the five retrotransposons in B. rapa was also smaller than those in B. oleracea. The genome size of B. oleracea is larger than that of B. rapa (Arumuganathan and Earle, 1991). Therefore, we hypothesized that a species having a larger genome size in Brassicaceae has more copies of retrotransposons with larger nucleotide variation. Increase of copy number can simply result in more mutations, because mutations in retrotransposons may be selectively neutral or nearly neutral. A significant positive correlation was observed between genome size and average number of substitutions in BoSTF7a homologs in nine diploids, but no correlation between them was observed in the Tto1 homologs. B. nigra and S. alba had exceptionally high percentages of non-functional Tto1 homologs, more than 80% (Table 5, Table 6), suggesting that Tto1 in B. nigra and S. alba might have independently lost the activity of transposition after speciation. One possible scenario of accumulation of inactivated retrotransposons in B. nigra and S. alba is that, after speciation, both species might have independently experienced reduction of effective population size, such as a bottleneck event, leading to fixation of retrotransposons in the various genome positions by chance, which should have been followed by accumulation of null mutations. This scenario might also explain the exceptional pattern of BoSTF7a of S. alba, which did not form a species-specific clade, although alternative hypotheses such as horizontal gene transfer or introgression cannot be ruled out. If these two species are excluded as exceptions, there was a significant positive correlation between genome size and the average number of substitutions of Tto1 homologs was obtained (r = 0.821, P = 0.0012, permutation test). These findings suggest that genome size of species, copy number of retrotransposons, and average number of substitutions in the retrotransposons correlate positively with each other.
Two retrotransposons may have contributed positively to the increase of genome size, in part, at least in the seven diploid species in Brassicaceae by amplification of their genome, supported by the phylogenetic patterns (Fig. 4, Fig. 5). Comparison of transposable elements between A. thaliana and B. oleracea has indicated that important factors involved in the genome size difference between B. oleracea and A. thaliana are proliferation of both class I and class II transposable elements and genome triplication in Brassica (Zhang and Wessler, 2004). There may be many transposons, such as the STF7a and Tto1 homologs, which contribute to the genome size expansion in Brassica.
The transposition of retrotransposons is activated in plants under stress conditions and in a hypomethylated condition (Grandbastien, 1998; Hirochika et al., 2000). Such a situation might contribute to the difference of copy number of retrotransposons among the nine diploids in Brassicaceae, which would have experienced various environmental conditions. It is of great interest whether the difference of copy number of the BoSTF7a and Tto1 homologs among the nine diploids was influenced by environmental stresses and epigenetic regulations. Since plant materials used in the present study consisted of only nine diploids in the tribe Brassiceae, further studies are needed to confirm the correlation between genome size and retrotransposon variations in different lineages.
Another evolutionary mechanism of the change of genome size is deletion of retrotransposons leading to decreased size. Larger and more frequent deletions, more than 40 times, in a non-LTR retrotransposon, Lau 1, have been observed in Drosophila melanogaster than in the Laupala cricket, whose genome is 11 times larger than that of D. melanogaster (Petrov et al., 2000). More frequent and larger deletions during double-strand break repair can be seen in Arabidopsis than in tobacco, which has a twenty-fold larger genome than A. thaliana (Kirik et al., 2000). Our analysis, however, showed no negative correlation between genome size and frequency of short deletions in the BoSTF7a and Tto1 homologs, but a positive correlation was observed between genome size and the number of deletions in the BoSTF7a homologs in the nine diploids. Reduction of genome size due to the loss of sequences was not observed in retrotransposons in Brassicaceae analyzed in the present study. However, S. alba and B. nigra had many inactivated retrotransposons and might be in the process of losing inactivated retrotransposons due to genetic drift.
The phylogenetic analysis of the present study showed that multiple species-specific clades were formed in the BoSTF7a homologs and the Tto1 homologs, the first being classified into the Ty3-gypsy group and the second into the Ty1-copia group in the diploid species. As well as these two retrotransposons, phylogenetic analysis also showed that species-specific clades were also formed in the sequences of three other Ty3-gypsy and one Ty1-copia retrotransposons in B. rapa and B. oleracea. Multiple clades of each species suggest the maintenance of the ancestral variation in their genomes. In other words, in ancestral species, some types of sequences might have existed and two or three types might have been fixed in a species, while other types might have been lost in that species. All clades contained multiple similar sequences derived from a given species, indicating that one or a few types of retrotransposons may have been amplified after speciation. Thus, a high rate of amplification and loss of retrotransposons is required to explain the pattern of the phylogenetic tree. It has been suggested that the Ty1-copia retrotransposons diverged before the modern plant orders arose because of their high sequence heterogeneity (Konieczny et al., 1991; Flavell et al., 1992a, b; Voytas et al., 1992; VanderWeil et al., 1993; Noma et al., 1997). In genus Vicia, Hill et al. (2005) have suggested the possibility that different retrotransposon groups have different levels of activities. Low heterogeneity in both Ty3-gypsy and Ty1-copia retrotransposons observed in the present study suggest that the Ty1-copia retrotransposons as well as the Ty3-gypsy retrotransposons differentiated after speciation of the diploid species in Brassicaceae.
In the allopolyploid species in Brassica, no species-specific clades were formed, their retrotransposons were clustered in the clades of their original genomes, indicating that the amplification and differentiation of the STF7a homologs and the Tto1 homologs took place before emergence of the allopolyploids. The nucleotide divergence of the two LTR sequences can be used for estimation of insertion time of LTR-retrotransposons (SanMiguel et al., 1998). Using the values of LTR sequence divergence, the insertion time of LTR-retrotransposons has been estimated to be within the last few million years in A. thaliana, barley, rice, tomato, and wheat (Bennetzen et al., 2005). The time of divergence between B. nigra and B. oleracea has been estimated to be a few million years ago (Yang et al., 1999). These estimations suggest that the amplification and differentiation of the STF7a and Tto1 homologs might have occurred after speciation of various monogenomic species in Brassicaceae.
We thank Dr. S. Tsuchimoto for his helpful comments and suggestions. This work was supported in part by a grant-in-aid (19208001) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
|