| Edited by Minoru Murata. Takashi Ryu Endo: Corresponding author. E-mail: trendo@kais.kyoto-u.ac.jp |
Supernumerary B chromosomes are present in many species of both plants and animals. Although dispensable, and extra to the host’s normal chromosomes, i.e., A chromosomes, B chromosomes are found in well over 1000 flowering plants, including both diploids and polyploids (Jones, 1995). In plants, B chromosomes enhance their transmission potential in a selfish way by a variety of drive mechanisms: premeiotic, meiotic and postmeiotic, with postmeiotic on the male side being by far the most common (Jones and Rees, 1982; Jones, 1991). Hasegawa (1934) first gave a cytological description of directed nondisjunction of the B chromosomes to the generative nucleus at the first pollen mitosis in rye. Müntzing (1945, 1946, 1948) later obtained variants of the rye B chromosomes, such as telosomes and isochromosomes of the short and long arms, and a long-arm deficiency, and he proved that there are sticking sites on either side of the centromere which prevent normal anaphase separation of the chromatids, and thereby cause the nondisjunction. However, nondisjunction did not occur in deficient B chromosomes, or in isochromosomes for the short arm, when either of these two types was present alone in the pollen. The process was inexplicable at first, but Lima-de-Faria (1962) later proposed that the sticking regions are present in both of these types, but are not active, and that a trans-acting factor present in the distal part of the long arm is needed to enable nondisjunction in deficient types as well as in the standard B chromosome itself. It is now known that what Lima-de-Faria described as the knob region (C-band) at the end of the long arm is actually comprised of a concentration of two families of B-specific sequences, E3900 and D100 (Sandery et al., 1990; Blunden et al., 1993; Langdon et al., 2000). The stickiness of the receptors adjacent to the centromere is now regarded as being controlled by a trans-acting element/gene located in these sequences (Jones and Puertas, 1993; Jones and Houben, 2003). A separation between the distal and proximal regions of the rye B is a prerequisite for studying the functions of these two regions. B-A translocations in rye M1 plants were induced by gamma irradiation, but none of these translocations were transmitted to the progeny (Hasterok et al., 2002). This highlighted the fact that B-A translocations are generally intolerable in diploid plants such as rye, although a rare spontaneous A-B translocation was reported in a rye strain (Schlegel and Pohler, 1994).
Rye B chromosomes introduced into common wheat retain the same pattern of nondisjunction behavior as exhibited in rye itself (Lindström, 1965; Niwa et al., 1997). This feature of the rye B provides an opportunity to dissect the B chromosome, for the first time, into numerous segments, using addition lines in hexaploid wheat, and exploiting chromosome mutations induced in wheat by unique alien gametocidal (Gc) chromosomes (Endo, 1990, 2007). When the gametocidal chromosome is present in a sporophyte, chromosome rearrangements occur in the gametes to which no gametocidal chromosome was distributed. This Gc system has been proved to be effective in inducing chromosomal mutations in rye and barley chromosomes added to common wheat (Endo et al., 1994; Shi and Endo, 2000; Endo, 2003). These induced deletions and translocations involving the alien chromosomes have been used for chromosome mapping of the alien chromosomes (Serizawa et al., 2001; Masoudi-Nejad et al., 2002, 2005; Nasuda et al., 2005; Ashida et al., 2007). In this study two types of gametocidal chromosome were introduced into common wheat lines carrying rye B-chromosomes of different origin, and high frequencies of rearrangements were induced in the B chromosomes. The rearranged B chromosomes lacking various segments were studied in terms of their capacity to undergo nondisjunction.
Two lines of common wheat (Triticum aestivum L. cv. Chinese Spring) carrying rye B chromosomes of different origin were used. The B chromosome in the first line was originally introduced into a Nepalese strain of wheat from a spring rye variety from Transbaikal, Siberia (Lindström, 1965; described as Bs in this paper), and then transferred into Chinese Spring. The B chromosome in the second line was derived from a Korean cultivated rye (Niwa et al., 1997; described as Bk in this paper). A plant of the first line carrying four Bs chromosomes was crossed as the female plant with a Chinese Spring line carrying a gametocidal chromosome derived from Aegilops triuncialis (3CSAT). The progeny that had one 3CSAT chromosome and two or four Bs chromosomes (Fig. 1A) were then self-pollinated, or hand-pollinated, with euploid CS pollen. All seeds on the selfed or crossed spikes were used for the screening for aberrant Bs chromosomes. Two plants of the second line carrying three or four Bks were crossed as the male parent to a Chinese Spring line carrying a gametocidal chromosome derived from Ae. cylindrica (2C). The progeny that had one 2C chromosome and one to four Bk chromosomes were self-pollinated, or hand-pollinated, with euploid CS pollen. The Bk chromosomes often failed to pair at the short arm (Fig. 1B). Shriveled seeds of the selfed plants, or crosses, were used for the screening for aberrant Bk chromosomes, since such seeds are generally expected to have chromosomal abnormalities.
 View Details | Fig. 1 FISH and GISH photographs of the standard and aberrant rye B chromosomes isolated in common wheat. FISH signals of the repetitive sequences D1100 and E3900 are shown in green, and GISH signals in red. Wheat chromosomes are in blue. (A) A mitotic metaphase of a common wheat line carrying a pair of rye Bs chromosomes and one 3CSAT chromosome. (B) A meiotic metaphase I of a common wheat line carrying a pair of rye Bk chromosomes and one 2C chromosome. (C) Standard (Bs) and aberrant (Bs-1 to Bs-10) Bs chromosomes. (D) Standard (Bk) and aberrant (Bk-1 to Bk-3) Bk chromosomes. (E) Bk and Bk-1 probed with the E3900 sequence. (F) Nondisjunction of Bk-2 at the first pollen mitosis. (G) A mitotic metaphase of a plant carrying two standard Bs (indicated with arrows) and three Bs-1 chromosomes (indicated with arrowheads). (H) A mitotic metaphase of a plant carrying six Bs-9 (indicated with arrowheads) and two Bs-10 (indicated with arrows) chromosomes. Scale bars = 10 μm. The vertical black bar is a scale for (C), (D) and (E). |
Root tips were treated in ice water for 18–24 h, and then fixed in 3:1 (ethanol: glacial acetic acid) fixative for a few days. The fixed root tips were stained in aceto-carmine and chromosome preparations were made by the squash method. Screening for aberrant B chromosomes was conducted by fluorescence in situ hybridization (FISH), using the B-specific subtelomeric repeat sequences D1100 and E3900 (Sandery et al., 1990; Blunden et al., 1993), and by genomic in situ hybridization (GISH) using total genomic DNA of rye as a probe. The D1100 sequence was cloned from a plasmid kindly provided by Dr. R. Hasterok, University of Silesia, Poland. The E3900 sequence was amplified by PCR using primer sets, 5’-TGAGCCTCGTCCTGAAACTT-3’ and 5’-GCGTAACCGGTGAACTCAAT-3’, and template DNA from CS plants carrying the Bk chromosomes. The total genomic DNA was extracted from the leaves of rye plants. The FISH and GISH probes were made by either direct labeling using FITC-11-dUTP (Roche) (green fluorescence) and rhodamine-5-dUTP (Roche) (red), or by indirect labeling using Biotin-High Prime Kit (Roche) and Dig-High Prime Kit (Roche). The digoxigenin- and biotin-labeled probes were detected with anti-digoxigenin-Fab fragment (green) and Cy3 Labeled Streptavidin (red), respectively. FISH and GISH was conducted as described by Hagras et al. (2005).
Table 1 shows the summary of the screening of rearranged B chromosomes by GISH/FISH analysis. Two deletions in the long arm of the B chromosomes were found in a total of 157 progeny plants of the disomic standard B addition lines with no gametocidal chromosome. The 3CSAT and 2C chromosomes induced structural changes in the B chromosomes in 16.1% and 7.5% of the progeny plants examined, respectively. Chromosome breakage occurred in both chromosome arms, and at the centromere. A total of 18 out of 54 plants carrying aberrant B chromosomes induced by the gametocidal chromosomes had multiple B aberrations. In most cases the aberrant B chromosomes were also found together with normal B chromosomes. Representative rearrangements of the B chromosomes are shown in Fig. 1C and 1D.
 View Details | Table 1 Frequency of aberrant B chromosomes that occurred spontaneously and that were induced by the gametocidal chromosomes |
The nondisjunction properties of the rearranged B chromosomes were examined by further cytological screening conducted to obtain plants carrying only aberrant B chromosomes in the subsequent generations. Consequently, ten aberrant Bs chromosomes were isolated in CS with no standard B present (Fig. 1C). Bs-1 was a Bs short-arm telosome; Bs-2 and Bs-8 were deletions in the long arm, but the former was possibly an isochromosome of the short arm; Bs-3 was a Bs long-arm isochromosome; Bs-4 was a deletion in the short arm; two translocations, Bs-5 and Bs-6 with breakpoints in the short arm, were also found in the same plant, probably originating from a reciprocal translocation with a wheat chromosome; Bs-7 was a translocation between Bs and a wheat chromosome 6B (identified by C-banding, photograph not shown); and Bs-9 and Bs-10, with breakpoints in the long arm, were probably reciprocal translocations with a wheat chromosome, because they were derived from the same plant. Two translocations between Bk and wheat chromosomes had translocation points within the repetitive sequences of the Bk long arm (Fig. 1D; Bk-1, Bk-2). All the rearranged B chromosomes, except Bk-2, were separated from the standard Bk chromosome, and were studied for their nondisjunction capacity in terms of their transmission frequency in the progeny. The nondisjunction properties of Bk-2 were investigated in the pollen grain mitosis of a plant carrying one Bk-2 and one standard Bk chromosome.
Transmission behavior of the standard Bs in the progeny of the disomic addition line indicated that the standard Bs also underwent nondisjunction in common wheat (Table 2). The transmission data also showed that none of the B chromosomes segregated regularly at meiosis of the disomic B addition lines, as described previously by Müntzing (1969). This was probably due to meiotic pairing failure at MI, especially at the short arm (Fig. 1B). Once distributed to meiocytes, the standard Bs underwent nondisjunction in half of the macrospores (11 out of 22), and exclusively in microsporogenesis (18 out of 19).
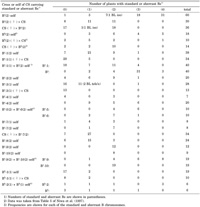 View Details | Table 2 Transmission of standard and aberrant rye B chromosomes in Chinese Spring (CS) |
The standard Bk retains its nondisjunction capability in common wheat (Niwa et al., 1997). The occurrence of nondisjunction of Bk is evident from the high incidence of plants with three or four Bks in the selfed progeny of the disomic Bk addition, and also from the high incidence of plants with two Bks in the backcrossed progeny of the disomic Bk addition (Table 2, data cited from Niwa et al., 1997).
The transmission data show that Bs-1, Bs-2, Bs-7, Bs-8, Bs-9, Bs-10 and Bk-1 did not undergo nondisjunction on their own (Table 2). The occurrence of single plants with three of the aberrant B chromosomes in the selfed progeny of the Bs-2 and Bs-8 disomic addition lines can be attributed to their irregular meiotic pairing, resulting in the production of gametes with two aberrant B chromosomes. Bk-1 had part of the D1100 sequences but completely lacked the E3900 sequences (Fig. 1E), and no nondisjunction was observed at the first pollen mitosis (12 pollen grains observed; no photograph shown).
The occurrence of one plant with three Bs-3 chromosomes in the selfed progeny of the monosomic Bs-3 addition indicated that Bs-3 underwent nondisjunction (Table 2). The high frequency of plants with three Bs-4 chromosomes (6 out of 20) in the selfed progeny of the disomic Bs-4 addition suggested the occurrence of nondisjunction of Bs-4 (Table 2). Three plants carrying four Bk-2 chromosomes, but no standard Bks were obtained in the selfed progeny of the double monosomic plant for the standard Bk and Bk-2 chromosomes (Table 2). This suggested that Bk-2 retained the capacity for nondisjunction, and nondisjunction of Bk-2 was also shown to occur in the first mitotic division of a microspore to which the standard Bk was not distributed (Fig. 1F).
The selfed progeny of a plant disomic for Bs-5 and Bs-6 contained six plants with three Bs-5 chromosomes and one plant with three Bs-6 chromosomes (Table 2). This result suggested that Bs-5 retained the nondisjunction capability on its own. Bs-9 and Bs-10 showed no nondisjunction behavior when they were separately added to common wheat; however, the number of Bs-9 increased up to four in the selfed progeny of a plant disomic for Bs-9 and Bs-10, although the number of Bs-10 stayed at two in all progeny (Table 2). The number of Bs-9 further increased to six in the selfed progeny of a plant tetrasomic for Bs-9 and disomic for Bs-10 (Fig. 1H). This result indicated that the nondisjunction of Bs-9 was evoked by the coexistence of Bs-10 in the same plant.
There were four plants with three Bs-1 chromosomes and 11 plants with two Bs-1 chromosomes in the selfed progeny (40 plants) of a plant monosomic for Bs-1 and disomic for the standard Bs (Table 2, Fig 1G). This indicated that the nondisjunction of Bs-1 was caused by the standard Bs coexisting in the same plant. On the other hand, the standard Bs chromosomes underwent nondisjunction less frequently when coexisting with Bs-1 than when they were by themselves (Table 2).
Rearranged Bs, such as telosomes, isochromosomes and terminal deletions were previously reported to occur spontaneously in euploid rye (Müntzing, 1945, 1946, 1948), and in this study also two deletions were observed in the progeny (157 plants) of the B addition lines of common wheat (Table 1). The addition of a gametocidal chromosome to the B addition lines of common wheat clearly raised the incidence of rearrangements in the Bs (54 out of 526 plants) (Table 1). Eighteen of the 54 plants also contained two or three aberrant Bs. High frequencies of large structural rearrangements were detected in an irradiated M1 generation of an experimental population of rye, but the M2 progeny had none of these rearrangements (Hasterok et al., 2002). On the other hand, various rearranged B chromosomes induced by the gametocidal chromosomes were established in common wheat (Fig. 1C and 1D). The translocations between the B and wheat chromosomes were also obtained for the first time in this study, due no doubt to the greater tolerance of polyploid wheat genome. No whole-arm translocation between the B and wheat chromosomes was found in this study, although they were frequently induced in barley chromosomes by the gametocidal chromosomes (Endo, 2003; Ashida et al., 2007). It is tempting to speculate that the B centromere has a unique structure that prevents centromeric fusion with the wheat centromere.
The occurrence of nondisjunction can be inferred genetically from the enhanced transmission of a supernumerary chromosome in the progeny, as compared with the numbers expected from regular gametogenesis. For example, if the selfed progeny of a plant monosomic or disomic for a supernumerary chromosome contained plants with three or four of the supernumeraries, it may safely be said that nondisjunction occurred for the supernumerary. Nondisjunction, on the other hand, can be judged not to have happened when supernumerary chromosomes occur in none or few of the progeny; the rare occurrence of the supernumerary can then be explained as a result of irregular meiotic segregation.
It has been suggested that centromeric B segments separated from the distal repetitive sequences of the long arm do not undergo nondisjunction in euploid rye (Jones and Puertas, 1993). The transmission data of the dissected B chromosomes in this study demonstrated that the centromeric regions of the B chromosome did not undergo nondisjunction in common wheat (Table 2: Bs-1, Bs-2, Bs-7, Bs-8, Bs-9). It was also proved in this study that the B-specific repetitive sequences translocated onto a wheat chromosome did not cause nondisjunction of the wheat chromosome (Table 2: Bs-10). The dissected Bs with deficiencies in the short arm, Bs-3, Bs-4 and Bs-5, retained the nondisjunction capability, but their nondisjunction frequency, especially that of Bs-3, was much lower than that of the standard Bs (Table 2). Two pericentric sticking sites, or receptors, are suggested to be present on the short arm and long arm (Jones and Puertas, 1993). Bs-3 was devoid of the sticking site on the short arm because it was a long-arm isochromosome, and Bs-4 and Bs-5 might have lost the sticking site, at least partially, on the short arm. Although it is difficult to judge from a single occurrence of a plant with three Bs-6 chromosome, Bs-6, the counterpart of Bs-5 in the reciprocal translocation, may have undergone nondisjunction when coexisting with Bs-5 (Table 2). The number or size of the sticking sites might regulate the incidence of nondisjunction.
The transmission data, and the observations on pollen mitosis, did not show that Bk-1 underwent nondisjunction. Bk-2 was proved to have the nondisjunction capability on its own, by the cytological observation at the first pollen mitosis (Fig. 1F). As was depicted by Jones and Houben (2003), the rye B chromosome has a trans-acting controlling element in the distal region comprising two types of repetitive sequences, two blocks of D1100 and one block to E3900 distal to the D1100 blocks. Bk-1 lacked the E3900 block completely (Fig. 1E) and appears to have lost one of the D1100 blocks (Fig. 1D). Bk-2 retained the D1100 blocks (Fig. 1D) and the E3900 block (photograph not shown). These facts indicate that some critical ‘gene’ is located within the region between the translocation points of Bk-1 and Bk-2. Alternatively, the amount of repetitive sequences themselves might be the critical factor for nondisjunction. The latter possibility is supported by a recent finding that both D1100 and E3900 are transcriptionally active (Carchilan et al., 2007).
Nondisjunction occurred in Bs-1 when it coexisted with the standard Bs, and in Bs-9 when it coexisted with Bs-10 (Table 2). These results clearly demonstrate, as already proposed (Jones and Puertas, 1993; Jones and Pašakinskienė, 2005), that nondisjunction of the rye B chromosome is controlled by two separate chromosomal sites, namely the long-arm distal region producing a certain trans-acting element, and the centromeric adhesion sites on which the element trans-acts. The adhesion sites appear to be in the pericentromeric regions (Fig. 1F), and are probably independent of the centromeric function, as demonstrated for the maize B chromosome (Han et al., 2007). The presence of such elements that react with the B centromere is also inferred from the fact that the nondisjunction frequency of the standard Bs chromosomes was decreased significantly when it coexists with Bs-1 (Table 2). The standard Bs might have competed with Bs-1 for the trans-acting gene product.
The gametocidal system has been demonstrated to dissect the rye B chromosomes rather readily, and in greater numbers of rearranged rye B chromosomes than can be otherwise isolated in common wheat. Such rye B chromosome dissection lines will have great utility in addressing the following questions on the mechanism of the nondisjunction of rye B chromosomes: Does the trans-acting control element for nondisjunction have a function only in gametophytes, or is it also active in sporophytes? Is nondisjunction regulated by the ratio of the amount of the trans-acting control element and the number of the centromeric adhesion sites? Exactly where are the trans-acting control element produced and precisely where are the adhesion sites located?
This article is a contribution (No. 596) from the Laboratory of Plant Genetics, Graduate School of Agriculture, Kyoto University, Japan.
|