| Edited by Minoru Murata. Chiharu Nakamura: Corresponding author. E-mail: nakamura@kobe-u.ac.jp |
Germination of plant embryo after imbibition (hydration) is a dynamic process requiring activation of a number of metabolic enzymes, which is accompanied by the rapid increase in oxygen consumption through mitochondrial respiration. There are reports showing that oxygen consumption starts and proceeds via the cytchrome pathway (cyt path) and this main electron transport path supplies enough energy for sustaining embryo germination and early growth of developing seedlings (Ehrenshaft and Brambl, 1990; Attucci et al., 1991). On the other hand, there are a number of reports describing that plant embryo can germinate normally in the presence of cyt path inhibitors like KCN and antimycin A (AA), but germination and subsequent seedling growth are severely suppressed by inhibitors of the alternative (alt) path including salicylhydroxamic acid (SHAM) and n-propyl gallate (nPG) (Yentur and Leopold, 1976). The engagement of the alt path in plants however has been postulated mainly by the perturbation of respiration by exogenously supplied inhibitors of the cyt and alt paths. Results of such pharmacophysiological study alone have been criticized for the non-specific inhibition of other oxidases, particularly by SHAM (Day et al., 1996). Although several studies addressed the issue of mitochondrial biogenesis in plants (Logan et al., 2001; Li-Pook-Than et al., 2004; Howell et al., 2006), a very little information is available at the gene expression and protein accumulation levels on the development of the respiratory paths during embryo germination and early seedling growth.
The cyanide insensitive and SHAM sensitive alt path, which is found in plants, some fungi, yeast and protozoa, branches from the main cyt path at the ubiquinol pool and consists of a sole protein (terminal oxidase) named alternative oxidase (AOX). AOX by itself does not contribute to the proton gradient formation and thus to ATP synthesis. Instead, the released energy of electrons passing through this path is lost as heat. AOX therefore is generally considered as an energy-dissipating bypass or an overflow conduit of the excess electrons when the cyt path is saturated (Lambers, 1982). Until now, biological function of AOX has been firmly established only for the specialized plant thermogenic tissues (Seymour et al., 2003). Additional hypotheses have been proposed to explain physiological roles of AOX (for reviews see, Millenaar and Lambers, 2003; Joseph-Horne et al., 2001; Veiga et al., 2003b). Notable ones include prevention of over-reduction of mitochondrial electron transport chain under conditions when growth is restricted such as low temperature (Purvis and Shewfelt, 1993), drought (Collier and Commins, 1992), high salinity (Hilal et al., 1998) and low supply of minerals (Parsons et al., 1999). AOX may also function in the maintenance of constant energy charge and in scavenging reactive oxygen species in mitochondria during growth under variable environment conditions (Moore et al., 2002) as well as in apoptosis and genetic necrosis (Vanlerberghe et al., 2002; Marechal and Baldan, 2002; Mizuno et al., 2005; Sugie et al., 2007).
We studied the development of the cyt and alt paths and transcript and protein profiles associated with mitochondrial biogenesis during germination and early seedling growth in wheat under normal and restricted conditions. Our results showed that both of the cyt and alt paths were operating and their capacity increased, which was coupled with increases in transcript and protein levels of these paths. Inhibition of the cyt path resulted in a marked increase in the alt path capacity that coincided with an increased level of AOX protein and its transcript, suggesting that alt path can support embryo germination and early seedling growth in conjunction with the complex I when the main cyt path was restricted.
A common wheat (Triticum aestivum L.) cultivar “Chinese Spring” was used throughout the experiment. Seeds were imbibed under tap water for 5 h and placed at 4°C for 15 h to promote synchronized germination. Seeds thus imbibed (at time 0) were placed individually on filter paper wicks (10 × 22 mm) that were fitted into glass test tubes (15 mm in diameter) containing 1 ml of 0.1% (v/v) Hyponex solution (N-P-K = 6-10-5, Hyponex, Osaka, Japan) and incubated for 3 days at 25°C with a 16-h photoperiod at a light intensity of 110–120 μmol photons m–2 s–1 provided by cool white fluorescent lamps (a standard condition). After the end of the first, second and third day of incubation, seedlings were dissected, separated into shoots and roots and their fresh weights were individually measured.
For respiratory measurement in dry quiescent embryos, 3 dissected embryos were directly placed in 2 ml of buffer solution (10 mM Mes-50 mM Hepes, pH 6.6) in a cell with a Clark-type oxygen electrode (Rank Brothers, Cambridge, UK). The rate of oxygen consumption was measured continuously for 1 h at 25°C in the dark. For respiratory measurement in germinating embryos and developing seedlings, imbibed seeds were placed on moist filter papers in petri dishes and incubated for 72 h under the standard condition. At the indicated times, germinating embryos and growing seedlings were removed, cut into appropriate sizes (10 to 20 mg in fresh weight) using a scalpel and vacuum infiltrated with the buffer solution for 2–3 min to allow sufficient penetration of oxygen and respiratory inhibitors into the tissues. The capacity of the cyt path was estimated by the rate of oxygen uptake inhibited by 1 mM KCN in the presence of 2 mM SHAM or 1 mM nPG, while that of the alt path by the rate of oxygen uptake inhibited by 2 mM SHAM or 1 mM nPG in the presence of 1 mM KCN.
AA at 10, 30, 100 and 200 μM was added to inhibit the cyt path and its effect on growth in the dark was studied. After the end of the 3-day test period, seedlings were dissected, separated into shoots and roots and their fresh weights were individually measured. For rotenone treatment (100 μM rotenone alone, and 100 μM rotenone plus 30 μM AA), seedlings were incubated for two days continuously in the dark. For studying the effect of AA on the development of the alt path capacity, seedlings were grown for 3 days in the presence of 30 μM AA in the dark. The rate of oxygen uptake was measured in buffer, in which the same concentration of AA as that added during germination and growth was supplemented prior to the measurement.
Respiration was also measured using 500 μg of a mitochondria-enriched fraction that was isolated from 2-day-old seedlings grown under the standard condition and from ones grown for one day under the standard condition plus one additional day with 30 μM AA in the dark. For isolation of the mitochondria-enriched fraction, tissues were homogenized in 1 ml of extraction buffer containing 50 mM Tris-HCl (pH8.0), 3 mM Na2-EDTA, 0.44 M mannitol, 1 mM 2-mercaptoetanol and 1% (v/v) protease inhibitor cocktail (Nacalai Tesque, Kyoto, Japan). After centrifugation at 3,000 × g for 5 min, the supernatant was re-centrifuged at 15,000 × g for 10 min. The mitochondrial pellet was dissolved in a small volume of buffer and used for respiratory measurement. Respiration buffer was supplemented with 1 mM ADP, 10 mM succinate, 1 mM NADH and 5 mM pyruvate in order to maximize the AOX capacity. Disulfiram (0.1 mM) was used to inhibit the alt path to avoid possible inhibition of lypoxygenase by SHAM.
Western blot analysis was performed as described by Ohno et al. (2003) using a total protein fraction isolated from dry and germinating embryos and seedlings grown either under the standard condition or under the restricted condition in the presence of AA. Mitochondria-enriched fraction also was used for western blot analysis. Protein concentration was determined using BIO-RAD protein assay dye reagent (Hercules, Ca, USA) with IgG as a standard. In both cases (total protein and mitochondrial protein), fractions (20 μg protein) solubilized by SDS were resolved through 12.5% polyacrylamide gel and electro-transferred onto nitrocellulose membrane, Hybond-C extra (GE-Healthcare, Piscataway, USA). After incubation with specific antibodies, protein blots were developed using a Vectastain universal ABC kit (Vector laboratories, Burlingame, California, USA) as described by the manufacturer. Experiments were repeated at least twice using independently prepared samples.
For detecting cytochrome C (Cyt C), monoclonal antibody raised against human Cyt C (clone 7H8; Santa Cruz Biotechnology, California USA) was used. Monoclonal antibodies against AOX (Elthon et al., 1989) and ATP synthase subunit A (ATPA) (Luethy et al., 1993) were gifts from T. Elthon (University of Nebraska, USA). Anti-NAD9 polyclonal antibody against the wheat protein (Lammatina et al., 1993) was a gift from J. M. Grienenberger (Strasbourg University, France). Monoclonal antibody against yeast core I subunit of cytochrome C oxidoreductase (Cyt C Red) was a gift from H. P. Braun (University of Hanover, Germany) and those against yeast cytochrome C oxidase (COXII) and ATP synthase subunit VI (ATP6) of petunia were gifts from C. Chase (University of Florida, USA). Pre-stained broad range molecular markers (Precision plus protein standards from Bio-Rad, Hercules, USA) were used to determine approximate molecular sizes of the protein subunits.
Dry and germinating embryos and seedlings grown either under the standard condition and under the restricted condition with AA were removed at the indicated times and immediately frozen in liquid N2. Total RNA was isolated according to the guanidium thiocyanate method (Cephasol RNA-I, Nacalai Tesque, Kyoto Japan). For quantitative reverse transcriptase PCR (qRT-PCR) analysis, 2 μg of total RNA was reverse transcribed with oligo-dT primer using the first strand cDNA synthesis kit, Rever TRA ACE (Toyobo, Osaka, Japan), and the resulting cDNA was PCR amplified using the following specific primers. The total template RNA for cDNA synthesis was treated with DNase I to remove contaminated DNA. All primers were selected using the Primer 3 software available at http://frodo.wi.mit.edu/. Primer sequences are shown in Table 1.
 View Details | Table 1 A list of primer sets for qRT-PCR analysis |
qRT-PCR was performed using Roche Light Cycler instrument running LIGHT Cycler software version 3.5 and Light Cycler Fast Start DNA MasterPLUS SYBR GREEN I kit in a 10 μl reaction mixture. All cDNA samples were diluted 10 times and 1 μl of every diluted sample was used as a template in all experiments. The amplification consisted of the following four programs – initial denaturation for 10 min at 95°C; amplification for 10 sec at 95°C, annealing for ca. 10 sec (primer dependent), and extension for 30 sec at 72°C with single data acquisition – 40 cycles; melting curve analysis starting from 60°C and slow heating to 95°C with transition rate of 0.1°C per second with continuous data acquisition. All experiments were repeated twice with cDNA isolated from independently prepared samples and each transcript analysis was repeated twice giving a total of four replications per treatment. For all primer pairs, PCR conditions were optimized to avoid primer–dimer formation. Absence of non-specific products was checked by melting curve analysis and confirmed by gel electrophoresis and ethidium bromide staining. Negative control that contained all the reaction components except for reverse transcriptase was included in the amplification to ensure no genomic DNA contamination. Initial amount of target RNA was calculated using the second derivative maximum function of the Light Cycler software. For all primer pairs, the efficiency of PCR reaction was determined by amplification of serial dilutions of reference cDNA samples as described by the manufacturer and small differences in the efficiencies were corrected. For each target gene, the level of its transcript was normalized to that of the actin gene by use of the standard curve method, as described in the User Bulletin no. 2 (Applied Biosystems). A value of 1.0 was then assigned to the gene(s) with the highest transcript level(s) in each of the developmental stage or treatment as calibrator(s) and the values of all other stages or treatments were calculated relative to it.
We first measured the ability of oxygen consumption by dry quiescent embryos that were directly excised from stored seeds. Oxygen consumption began with an initial lag period of less than a few minutes after dry embryos were contacted with buffer solution, and its rate increased to ca. 0.3 nmol O2 min–1 mg–1 of fresh weight (fw) within 30 min to 1 h (Fig. 1A). This rapid respiratory burst agreed with the earlier reports in maize and rice embryos (Ehrenshaft and Brambl, 1990; Howell et al., 2006). We then monitored the time course of respiratory path development after imbibition for 72 h under the standard condition. Shoot and root growth continued for 3 days (Fig. 1B). The rate of total oxygen consumption rapidly increased to 0.49 nmol O2 min–1 mg–1 fw within 24 h, and further increased to 0.55 nmol O2 min–1 mg–1 fw at 72 h (Fig. 1A).
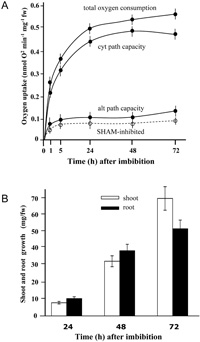 View Details | Fig. 1 Development of respiratory path capacities and seedling growth during the 3-day period under the standard condition. (A) Total oxygen consumption and estimated capacities of the cyt and alt paths in dry and germinating embryos and seedlings. A dotted line indicates the alt path activity estimated by the rate of oxygen consumption inhibited by 2 mM SHAM alone, indicating the alt path operation. (B) Shoot and root growth. |
We estimated in vivo capacities of the two respiratory paths using respiratory inhibitors. The capacity of the cyt path increased to reach 0.47 nmol O2 min–1 mg–1 fw at 48 h (Fig. 1A). The alt path capacity also increased within 5 to 24 h to reach 0.14 nmol O2 min–1 mg–1 fw at 72 h. These values are similar to the reported capacities of the two paths in germinating embryos in several monocotyledonous plant species (Millenaar et al. 2001). The estimated capacity of the cyt path was about 3 to 4.5-fold higher than that of the alt path throughout the tested period, confirming that the cyt path was the major path during this critical stage. The rate of oxygen consumption inhibited by the addition of 2 mM SHAM alone or 1 mM nPG also (data not shown) increased till 5 h and thereafter remained fairy constant. Although such value did not give a direct estimate of the alt path activity, it indicated that the alt path was operating in germinating embryos and growing seedlings.
The burst of respiration after imbibition suggested that required components of the cyt and alt paths were preserved in dry quiescent embryos and they immediately responded to imbibition. We thus studied protein levels of at least one subunit of all the respiratory complexes involved in proton pumping as well as those of Cyt C and AOX. Western blot analysis using specific antibodies showed that the protein components tested in the complexes I (NAD9) and III (Cyt C Red) and Cyt C were present at substantial levels in dry embryos and their amounts remained constant during the 3-day test period when the protein levels were estimated on the total protein basis (Fig. 2A).
 View Details | Fig. 2 Profiles of mitochondrial protein subunits of the two respiratory paths in dry and germinating embryos and seedlings. Specific antibodies were used in the western blot analysis. (A) Total proteins (20 μg) were applied in the gels. (B) Crude mitochondrial proteins (20 μg) were applied in the gels. The experiments were repeated for 2 to 4 times depending on the proteins. |
By contrast, the amount of two ATP synthase subunits in the complex V (ATPA and ATP6) showed an steady increase towards the later stages until 72 h. Since the levels of protein components measured on the total protein basis might not give direct information on the number of mitochondria per cell, we next studied the protein levels on the crude mitochondrial protein basis (Fig. 2B). The level of ATP6 thus measured showed a dramatic increase after 47 h, agreeing with the result obtained on the total protein basis. Cty C Red and cyt C also showed apparent increases (Fig. 2B). It was reported in maize embryos that there were no changes in mitochondrial DNA copy number during the initial 47 h of germination, suggesting no significant changes in mitochondrial number per cell at this stage (Logan et al., 2001). The observed increase thus was likely due to increased synthesis of these component proteins within mitochondria, indicative of active mitochondrial biogenesis in terms of mitochondrial fuction. Taken together, the observed protein levels and respiratory capacity indicated that dry wheat embryos contained all components of the cyt path complexes and cyt C as much as needed for the oxidative phosphorylation to begin upon imbibition. The result also suggested that de novo synthesis of these component proteins took place during embryo germination and seedling growth. The level of COXII could not be studied because the monoclonal antibody against yeast COXII failed to detect any protein bands in all tissues examined.
AOX protein was also present in dry embryos but its level remained fairy constant throughout the 3-day test period when measured on the total protein basis (Fig. 2A). The result was consistent with the observed constant capacity of the alt path after 24 h (Fig. 1A). AOX was likely activated upon imbibition similar to the cyt path components. It is known that plant AOX can exist either as an active monomer or as a less active covalently associated dimer. Since we extracted mitochondrial and total proteins using reducing buffer, we detected only a single monomeric band corresponding to the reduced form of AOX. The apparent molecular mass of this monomeric form on the polyacrylamide gels was about 40 kDa, which was higher than 37 kDa deduced from the cDNA sequences of the two wheat Aox genes (Takumi et al., 2002). A similar size discrepancy was reported in soybean (Finnegan et al., 1997).
To validate the assumption that the required protein components are not only stored in dry embryos at sufficient quantities but also synthesized de novo after imbibition, we studied changes in the transcript levels of representative genes in the cyt and alt paths by qRT-PCR analysis. Since COXII protein could not be studied by western blotting analysis, we added cox2 gene in addition to nad7, cob and rps7 in quantification by qRT-PCR. Fig. 3 shows the relative transcript abundance of individual genes that was normalized by assigning the value of 1 to the gene(s) with the highest level in each of the four developmental stages.
 View Details | Fig. 3 qRT-PCR analysis of transcripts encoding mitochondrial protein components in dry and germinating embryos and seedlings. Transcript profiles of 5 mitochondria-encoded subunits (nad7, cob, cox2, atp6 and rps7) and 3 nuclear-encoded and mitochondria-targeted genes (Mn-SOD, TaMRPL5, Waox 1a) were analyzed using samples grown under the standard condition. Each data point represents the mean±standard deviation from two independent experiments. |
The analysis showed that dry embryos contained transcripts of all examined genes encoding protein components in the complexes I (nad7), III (cob), IV (cox2) and V (atp6). The results strongly suggested that these transcripts represented the ones synthesized during embryogenesis and stored in dry embryos. Transcript of nad7 was most abundant in dry embryos and its relative amount decreased thereafter. Sequential and differential expression was also apparent among all the genes examined after imbibition. Thus the relative abundance of atp6 and cox2 transcripts increased continuously, while the levels of cob and rps7 transcripts increased until day 2 followed by decreases. The profile of atp6 transcript agreed very well with that of the corresponding protein subunit (Fig. 2).
With respect to AOX transcript, we could study only that of Waox1a, one of the two functional AOX gene paralogs in wheat (Takumi et al., 2002; Naydenov et al., 2005), because non-specific amplification occurred in case of another paralog Waox1c (data not shown). Agreeing with the protein level (Fig. 2), relative abundance of Waox1a transcript remained at a constant and low level throughout the tested period (Fig. 3). Since in wheat no sequence information is available for nuclear encoded respiratory subunits other than AOX, we studied two additional nuclear genes encoding mitochondrial protein components, Mn-SOD and TaMRPL5, to check the nuclear transcription. Contrasting profiles were observed in these components, i.e., the amount of Mn-SOD transcript decreased during the first day and increased again after day 2, while TaMRPL5 transcript showed opposite changes. These results suggested that both mitochondrial and nuclear genes encoding mitochondrial protein components were differentially transcribed in germinating embryos and growing seedlings. Howell et al. (2006) have recently studied transcript levels of a set of mitochondrial genes and reported that cox2 gene peaked in 2 day post-germination in rice. A similar expression pattern was found in wheat atp6 in this study and a gene encoding F1α subunit in rice (Howell et al., 2006). The amount of rice Aox1a transcript quickly increased during the first day after imbibition, and then it stayed fairly constant similar to wheat Waox1a. The amount of wheat nad7 transcript initially decreased to reach a constant level (about a half of the initial level) during the second to the third day, whereas that of rice nad9 transcript showed an opposite trend during these periods. Expression profiles of cob genes in rice and wheat also differed significantly.
Since we observed that dry embryos begun respiration almost immediately after imbibition and the cyt path was the main contributor (Fig. 1A), we studied effects of the cyt path inhibitor AA on embryo germination and early seedling growth to estimate in vivo contribution of the alt path under conditions where the cyt path was restricted. To avoid complications that might arise from the photosynthetic activities, we performed this bioassay and related gene expression analysis using materials grown in the continuous darkness to ensure that energy was primarily supplied by oxidative phosphorylation. AA was applied at different concentrations, and fresh weights of both shoots and roots were measured after 3-day incubation. Application of AA did not affect embryo germination up to 200 μM, but it significantly inhibited seedling growth (Fig. 4A and B). The inhibitory effect of AA on shoot growth appeared to be concentration dependent but that on root growth reached a plateau level at 10 μM. The more severe inhibitory effect of AA on root growth might be ascribed to the fact that roots were in direct contact with the inhibitor and thus the inhibitor was incorporated from roots. An alternative explanation can be that roots are more vulnerable to the cyt inhibitors than shoots. Sustained seedling growth, although reduced, under the presence of AA suggested that the alt path could partly support seedling growth when the cyt path was inhibited.
 View Details | Fig. 4 Effects of respiratory inhibitors of the cyt path on shoot and root growth of germinating embryos and seedlings. Fresh weights of shoots (A) and roots (B) were separately measured at the end of the 3rd day after the start of the experiment (see Materials and Methods). LD: control under 16 h D (dark) and 8 h L (light); D: control under 24 h D; in the presence of 10, 30, 100 and 200 μM AA under 24 h D. (C) Effects of rotenone (Roth., 100 μM) alone and Roth. plus AA (30 μM) on total growth for two days. C: control without Roth. Bars indicate standard deviations (n = 5). |
To check the effect of full inhibition of the complex I, we incubated wheat seedlings for two days in the dark with 100 μM rotenone and also with combination of 100 μM rotenone plus 30 μM AA (Fig. 4C). Under this condition, rotenone alone greatly inhibited wheat embryo germination and growth. Nevertheless, the observed inhibited growth indicated that the complex I inhibition was bypassed likely by the activities of alternative NAD dehydrogenases (Rasmunson et al. 2004). It has been reported that a tobacco mutant lacking functional complex I can grow albeit at a much lower rate than the normal plants (Vidal et al. 2007). But the combined action of both rotenone and AA, which was assumed to result in the total inhibition of cyt path, did not allow any germination and growth.
The AOX capacity was reported to increase due to its increased synthesis after addition of KCN or AA (Clifton et al. 2005). To further explain the sustained embryo germination and seedling growth under the presence of AA, we studied changes in the alt path capacity in germinating embryos incubated under this restricted condition. The total respiratory activity and the capacity of the alt path were measured in germinating embryos incubated with and without 30 μM AA in the dark. AA was added for 24 h to one-day-old embryos that were germinating without inhibition. Under this condition, substantial increases were observed in both the total respiratory oxygen consumption and the alt path capacity compared to the normal non-inhibited condition (Fig. 5A). Measurement of the alt path capacity using disulfiram in crude mitochondrial fractions prepared from the non-treated and AA-treated seedlings also revealed a significant increase in the AA-treated samples as compared to the non-treated ones (Table 2). These results suggested that the increased total activity was due to the increased AOX activity.
 View Details | Fig. 5 Effect of AA on the alt path capacity and the transcript and protein amounts in germinating embryos and seedlings. (A) Total respiratory activity and the estimated capacity of the alt path in germinating embryos incubated in the dark. Data were from three independent measurements. (B) Protein subunits of the complexes III (cyt C Red) and V (ATPA), and AOX detected by western blot analysis. The blot was normalized to cyt C Red, which showed stable levels. Growth condition was the same as in (A). The intensity of AOX band was measured using GS-710 Imaging Densitometer (BioRad) and spot intensities were quantified by Image J (available at http//rsb.info.nih.gov/ij). The band intensity of non-treated control was set to 1 and AOX level in AA treated sample was calculated relative to the control. (C) qRT-PCR analysis of transcripts of the mitochondria (nad7, cob, cox2, atp6 and rps7) and nuclear encoded (Mn-SOD, TaMRPL5 and Waox1a) genes. +AA: One-day-old embryos germinating under the standard condition were incubated for additional two days in the presence of 30 μM AA in the dark. –AA: unrestricted samples grown for 3 days in the dark. |
 View Details | Table 2 Alt path capacity measured in a crude mitochondrial fraction extracted from 2-day-old seedlings |
The amounts of AOX protein and its transcript showed substantial increases under the restricted condition. An increase in protein abundance measured by densitometry in the treated sample was ca. 5-times more than the control (Fig. 5B). By contrast, the amount of protein subunits in the complexes I (NAD9, data not shown), III (cyt C Red) and V (ATPA) did not show significant changes. We compared the relative amount of Waox1a transcript by qRT-PCR analysis using the dark-grown 3-days-old samples that were incubated for the last two days with and without 30 μM AA in the dark. qRT-PCR analysis showed a dramatic increase in the relative amount of Waox1a transcript as compared to the non-restricted seedlings (Fig. 5C). Under the restricted condition, all transcripts other than that of nad7 significantly decreased their relative abundance. It was also shown that the transcript profile observed in the dark-grown 3-days-old samples was quite similar to that in the samples grown under the standard 16 h light and 8 h dark condition (Fig. 3).
The process of mitochondrial biogenesis during embryo germination and early seedling growth remains unknown in many aspects. We studied this important process based on the respiratory development and mitochondrial transcript and protein profiles. Our results showed that dry quiescent wheat embryos had the capacity of activating respiration through both the cyt path and the alt path immediately upon imbibition (Fig. 1). Because no apparent increases occurred in the amount of most protein complexes examined during the initial hours of germination (Fig. 2A), the observed respiration burst during this period can be ascribed to the presence of active protein complexes and/or to the rapid assembly of the preserved components as suggested by Attucci et al. (1991). As shown in the previous study by mitochondrial macroarray (Khanam et al., 2007) and the present study by qRT-PCR, dry quiescent embryos contain transcripts of all mitochondria- and nuclear-encoded genes needed for mitochondrial biogenesis (Fig. 3). Both studies revealed differential accumulation of individual transcripts in germinating embryos and developing seedlings, thus suggesting their de novo transcription. The accumulation of mitochondrial transcript was sequential, i.e., cob transcript showed a maximum level after 24 h, followed by those of cox2 and rps7 after 48 h and atp6 after 72 h (Fig. 3). Logan et al. (2001) reported that transcripts of all tested mitochondria-encoded genes were present in dry maize embryos and their amounts increased at 24 to 48 h after imbibition. Early transcription of the gene encoding ATP9 was reported in germinating maize embryos (Ehrenshaft and Brambl, 1990). Li-Pook-Than et al. (2004) studied mRNA accumulation of several selected mitochondrial genes in germinating wheat embryos. They reported that transcripts of mitochondria-encoded respiratory chain genes (nad7, coxI, coxII and atp6) were stably present during germination per mitochondria basis, while ribosomal protein genes (rps2, rps3 and rps7) showed lower steady-state levels in the later stages of development. We noted apparent increases in the amount of cob, cox2 and atp6 transcripts (Fig. 3). The amount of ATPA and ATP6 proteins also showed significant increases during the later stages (Fig. 2). These results suggest that active mitochondrial biogenesis takes place at the levels of both transcription and translation in wheat embryos after imbibition.
Blocking the cyt path by 200 μM AA did not affect embryo germination, although AA significantly inhibited seedling growth, particularly that of roots (Fig. 4A and B). Since AA at 10–30 μM has been commonly used in long-term (2–4 days) experiments to effectively inhibit plant tissue and cell respiration via the cyt path, our result indicates that wheat embryos can germinate even when the cyt path is severely inhibited. SHAM also inhibited seedling growth greatly when imbibed seeds were incubated with 2 mM SHAM for one day either during the first, second or third day after germination as compared with the uninhibited condition. SHAM inhibition was the greatest (nearly 50% for shoot growth and 80% for root growth) during the second day when the seedling growth rate was the greatest (data not shown). Although SHAM could non-specifically inhibit oxidases other than AOX (Day et al., 1996), this suggests that the alt path is operating and supporting seedling growth together with the main cyt path. The rate of total oxygen consumption showed a marked increase when the cyt path was inhibited by AA treatment as compared with the uninhibited level (Fig. 5A). This increase was apparently due to the increased alt path capacity coupled with the enhanced AOX at the both protein and transcript levels under this restricted condition (Fig. 5B and C). The up-regulation of the total respiratory capacity associated with that of the alt path capacity when the cyt path was inhibited was already shown in tobacco and Arabidopsis (Vanlerberghe et al., 1997; Saisho et al. 2001).
Sustained wheat embryo germination and seedling growth by enhanced AOX capacity suggests that protons are pumped at the complex I using electrons from intra-mitochondrial NADH derived from the active TCA cycle enzymes. In yeast strains that possess functional complex I, the induced AOX activity can support cell growth in the presence of cyt path inhibitors without any visible adverse effects (Veiga et al. 2003a). Rotenone, the complex I inhibitor, strongly inhibited wheat seedling growth and rotenone plus AA completely inhibited it (Fig. 4C). We in fact observed that dry and germinating wheat embryos contained the complex I components both at the protein and transcript levels (Fig. 2 and Fig. 3), although we could study only a part of the components. Taken together, our results suggest that operation of the alt path can help the complex I to continue proton pumping under the restricted conditions, and thus the alt path can at least serve as a safeguard for energy conservation in conjunction with the complex I when the cyt path is restricted during wheat embryo germination and early seedling growth. A causal relationship between the restriction of the cyt path under natural conditions and the enhancement of the alt path has to be further studied.
We thank Drs. T. Elthon, J. M. Grienenberger, H. P. Braun and C. Chase for their gifts of the antibodies. The work was supported in part by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to C. N., no. 13306002). N. G. N. is a recipient of Japanese Society for the Promotion of Science Postdoctoral Fellowship (grant no. P05187) and S. K. is a recipient of Japanese Ministry of Education Predoctoral Scholarship.
|