| Edited by Minoru Murata. Takashi R. Endo: Corresponding author. E-mail: trendo@kais.kyoto-u.ac.jp |
Rye (Secale cereale L.) is a major crop in many areas of Central America, Northern and Eastern Europe, and across Asia. Compared to other winter cereals, rye has an excellent tolerance to low temperatures and the ability to achieve relatively high grain yields under soil conditions in which other crops perform poorly (Madej, 1996). Rye is also important as a genetic source for wheat improvement programs, and it is a constituent of triticale, a synthesized crop. Especially, the short arm of chromosome 1R (1RS) of rye carries several important disease resistance genes that confer resistance to rust (Sr31, Lr26, and Yr9) and powdery mildew (pm8) (Singh et al., 1990). Although 1RS has contributed important disease resistance genes, wheat cultivars carrying this chromosome arm were shown to produce grain with quality defects, in particular, sticky dough and a reduction in dough strength (Martin and Stewart, 1990; Graybosch et al., 1993). The reduction in dough strength is thought to be due to the presence of monomeric secalins from rye (omega secalin) and the substitution of glutenins and gliadins (Dhaliwal and MacRitchie, 1990). In an attempt to eliminate or reduce these deleterious effects on grain quality, studies were undertaken to induce homoeologous recombination between chromosome arm 1RS of ‘Imperial’ rye and 1DS of wheat (Koebner and Shepherd, 1986; Koebner et al., 1986; Rogowsky et al., 1991; Lukaszewski, 2000).
The identification and intrachromosomal mapping of genes responsible for important agronomical traits is one of the main tasks of rye genetics, and to that end, well-saturated molecular linkage maps of rye, which are reliable and easy in application, are required (Khlestkina et al., 2004). Physical (cytological) maps of rye would complement the linkage maps. Physical maps of common wheat were constructed with an array of chromosome deletion stocks generated by the gametocidal system (Endo and Gill, 1996). The gametocidal system is a genetic system in which chromosome mutations are induced by unique alien chromosomes called gametocidal chromosomes (Endo, 1990, 2007). When added to common wheat in the monosomic condition, the gametocidal chromosome induces chromosomal mutations in gametes to which the gametocidal chromosome is not distributed, and the gametes with rearranged chromosomes either become lethal or survive to transmit the rearranged chromosomes to the progeny. The deletion stocks of common wheat were used to map 8241 expressed sequence tags (ESTs) in wheat (http://wheat.pw.usda.gov/NSF). Although chromosome deletions are supposed to be mostly lethal in diploid Triticeae species, such as rye and barley, deletions can mostly be maintained in hexaploid common wheat with trebled genomes (Endo and Gill, 1996). The gametocidal system can be used to induce rearrangements in rye chromosome 1R (Endo et al., 1994; Friebe et al., 2000; Masoudi-Nejad et al., 2002) and barley chromosomes 5H (Ashida et al., 2007) and 7H (Masoudi-Nejad et al., 2005; Nasuda et al., 2005) in the genetic background of common wheat. The common wheat lines carrying dissected 5H or 7H chromosomal segments proved useful in mapping EST markers. Ashida et al. (2007) demonstrated that the mapped 5H EST markers could be used in turn in screening critical plants carrying specific 5H segments by PCR, without cumbersome cytological screening, from the progeny of hemizygous lines carrying the 5H segments.
Deletion mapping should also be practicable for rye chromosomes with rye-specific DNA markers. There are some DNA markers specific to and ubiquitous on rye chromosomes (McIntyre et al., 1990; Katto et al., 2004), which are useful in identifying rye chromosomes or chromosomal segments incorporated into the wheat genomes. However, there are not many PCR-based DNA markers that can be used for chromosome identification (Lee et al., 1993, 1995) and mapping (Ma et al., 2001; Nagy and Lelley, 2003). In Triticeae, unlike in rice, the genome sequencing is not yet so advanced as to allow us to design PCR markers that are polymorphic between rye and wheat. Nevertheless, the kinds and the orders of genes corresponding to the chromosome fragments are highly conserved between rice and Triticeae, known as synteny, and this synteny enables us to predict Triticeae genome information from rice genome (Gale and Devos, 1998; Sorrells et al., 2003).
Anticipating a sharp increase in the number of available 1R-specific DNA markers in future, we applied the gametocidal system to develop an array of dissection lines of chromosome 1R, which can be used for physical mapping of chromosome 1R. Concurrently, we developed some PCR-based 1R-specific EST markers with which we can screen the 1R dissection lines for critical plants carrying the specific rearranged 1R chromosomes.
We used a common wheat (Triticum aestivum L., 2n = 6x = 42, genome formula AABBDD) cultivar Chinese Spring (abbreviated CS), a rye (Secale cereale, 2n = 2x = 14, RR) cultivar Imperial, wheat-rye addition lines of CS carrying a pair of Imperial rye chromosomes 1R (1Ri), its short arm (1RiS) and its long arm (1RiL), respectively, and a CS line carrying a pair of 2C chromosomes. The 2C chromosome is a gametocidal chromosome introduced into common wheat from Aegilops cylindrica (2n = 28, CCDD) and induces chromosomal structural changes in gametes in which the 2C chromosome is not present (Endo, 1988). We obtained these wheat lines from National BioResource Project, Japan (NBRP-Wheat). We crossed the 1R addition line (2n = 44, CS+1”1Ri) to the 2C line (2n = 44, CS+1”2C) and then backcrossed the F1 (2n = 44, CS+1’1Ri+1’2C) to the 1Ri addition line to obtain 45-chromosome (CS+1”1Ri+1’2C) plants. We cytologically screened the backcrossed progeny for rearranged 1Ri chromosomes.
We used simultaneous fluorescence in situ hybridization (FISH) with a rye-specific sequence pSc200 and genomic in situ hybridization (GISH) with total rye genomic DNA for the screening. The pSc200 sequence, a 521-bp satellite DNA sequence that is localized in tandem arrays in the terminal regions of all rye chromosomes (Vershinin et al., 1995, 1996), makes prominent landmarks of both distal ends of chromosome 1R. Chromosome preparation and probe labeling were conducted as described by Schubert et al. (1998). The in situ hybridization was performed as described by Endo et al. (2008).
We used 96 pairs of EST primers that were designed by the PCR-based Landmark Unique Genes (PLUG) system (Ishikawa et al., 2007) and that were supposed to be specific to wheat group-1 chromosomes (1A, 1B, 1D). We acquired them from a collection of wheat PLUG primers developed in Green Technology Project DM-1305, the Ministry of Agriculture, Forestry and Fisheries of Japan (unpublished). Each of the primer pairs was designed within separate exons of a wheat EST sequence that were derived from an orthologous rice genomic sequence on rice chromosomes 5 and 10, which have synteny with wheat chromosome group 1. These PLUG primer sets were expected to amplify 1R-specific PCR products because rye 1R and wheat group-1 chromosomes are known to have a high synteny (Gale and Devos, 1998; Feuillet and Keller, 2002) and because it is highly possible that the intron sequences of orthologous sequences in rye are different from those in wheat.
We also used 61 sets of primers of rye EST sequences that were obtained from the BLAST homology search and alignment. For designing the EST primers, we used EST consensus contigs developed in EGENES (Masoudi-Nejad et al., 2007) with the EST assembling strategy in EGassembler (Masoudi-Nejad et al., 2006). Briefly, All the rye ESTs (9194) were downloaded from the dbEST of NCBI and assembled into 1354 contigs and 4169 singletons. The BLAST homology search were conducted between these contigs and singletons, and all the wheat ESTs mapped to group-one chromosome (3803) from NSF (National Science Foundation, US) Wheat EST Mapping consortium (1AL: 752, 1AS: 383, 1BL: 865, 1BS: 526, 1DL: 886, and 1DS: 391). Rye- specific primers were designed in non-homologous segments in the 3’ end of the rye EST sequences where there were gaps or mismatches between query (rye) and subjects (wheat).
In addition we used two published primer sets, one for the Sec1 gene (Shimizu et al., 1997) and IAG95 (Mago et al., 2002). Table 1 shows the sequences of the primer sets that turned out successful in detecting rye segments in common wheat.
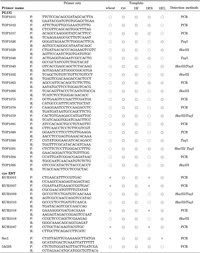 View Details | Table 1 PCR amplification using the PLUG (TOP) and rye EST (KUR) primers (○: amplification of PCR product, ◎: amplification of rye-, 1Ri-, 1RiS-, or 1RiL-specific PCR product, and ×: no amplification) |
We extracted genomic DNA from fresh or frozen young leaves by the modified CTAB method (Saghai-Maroof et al., 1984) and used 2 μl of the DNA solution (ca. 100 ng) as a template for PCR. The PCR mixture consisted of 2.5 μl of 10 × PCR buffer, 2 μl of dNTP (2.5 mM each), 1.5 μl of MgCl2 (25 mM), 0.5 μl of primers (10 pmol/μl), 0.12 μl of ExTaq Polymerase (5U/μl, TaKaRa), and 15.88 μl of dH2O. We conducted thermal cycling with an iCycler (BioRad) under the following conditions: For PLUG primers, 95°C for 5 min, 32 cycles of PCR (95°C for 30 sec, 58°C for 30 sec, and 72°C for 2 min), and 72°C for 7 min; for rye EST primers, 94°C for 2 min, 5 cycles (94°C for 30 sec, 65°C for 30 sec (with the temperature subsequently decreased 1°C per cycle), and 72°C for 1 min), and 35 cycles of PCR (94°C for 30 sec, 60°C for 30 sec, 70°C for 1 min), and 70°C for 7 min. We also digested the PCR products with restriction enzymes (HaeIII and TaqI, TaKaRa) to obtain polymorphism between rye and wheat. We checked the PCR products by agarose (Agarose S, Nippon gene) gel electrophoresis at 100 V for 50 min.
We cytologically screened the selfed progeny (572 plants) of the 45-chromosome (CS+1”1R+1’2C) plants and found 106 plants carrying structurally rearranged 1Ri chromosomes. The frequency (18.5%) of the occurrence of plants carrying rearranged 1Ri chromosomes was as high as those for other alien chromosomes (Endo, 2003). Examining the progeny of those plants, we established 55 CS lines carrying single aberrant 1Ri chromosomes in either hemizygous or homozygous condition. We designated these lines as ‘1Ri dissection lines’. FISH/GISH images of the rearranged 1R chromosomes are shown in Fig. 1, and their cytological characteristics are summarized in Table 2. There were 41 deletions, and 39 of them had single breakpoints and two (1Ri-8, 1Ri-20) had double breakpoints. There were 14 translocations between 1Ri and wheat chromosomes: Two of them had the rye centromere (wheat chromosome segments translocated onto the 1Ri chromosome), five had the wheat centromere (1Ri chromosome segments translocated onto wheat chromosomes), seven were whole-arm translocations (Robertsonian type), and the remaining one with the wheat centromere (1Ri-60) contained an interstitial 1RiL segment (confirmed by the subsequent PCR analysis). In summary, we identified 58 breakpoints in the 1Ri chromosome: 29 in the 1RiL arm, 10 in the 1RiS arm excluding the satellite, 4 in the satellite, and 15 in the centromere. The frequency of the breakpoints along the 1Ri chromosome seems to reflect the relative lengths of 1RiL (61.5%), 1RiS without the satellite (23.2%), the satellite (7.8%), and NOR (7.5%), but the breakage in the centromere was more frequent for its relatively small size. These breakpoints will make good diagnostic cytological landmarks of chromosome 1Ri in mapping DNA markers and might be useful in aligning BAC clones.
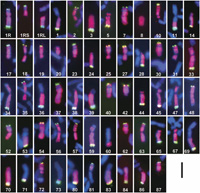 View Details | Fig. 1 Rearranged 1Ri chromosomes in the 1Ri dissection lines. The serial numbers in white represent the line numbers in Table 2. The pink GISH signals show rye chromatin and the green FISH signals show the pSc200 repetitive sequences. Bar = 10 μm. |
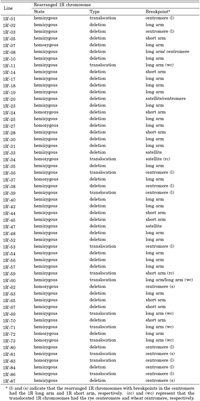 View Details | Table 2 1R dissection lines and rearranged 1R chromosomes |
We tested the 96 PLUG primer sets in PCR amplification with the templates of the euploid lines of wheat and rye, and the 1Ri, 1RiS and 1RiL addition lines. Nine (9.4%) of them amplified rye-specific products and additional ten (10.4%) generated rye-specific bands after the digestion with the restriction enzymes. Fourteen of these 19 PCR bands were allocated to 1RiL, three were to 1RiS and two were to both chromosome arms (Table 1). Of the remaining 77 primer sets, 57 amplified PCR products of wheat were indistinguishable in size from those in rye even after restriction enzyme digestion, 13 amplified PCR products in wheat but not in rye, and 7 amplified PCR products neither in wheat nor in rye. We also tested the 61 rye EST primer sets likewise and found that four (6.6%) of them amplified rye-specific PCR products, and additional three primer sets (4.9%) amplified PCR products that split into rye-specific bands after the digestion with the restriction enzymes. These seven rye EST primer sets all amplified 1RiL-specific PCR bands (Table 1). Of the remaining 54 primer sets, 28 amplified monomorphic PCR products in rye and wheat even after restriction enzyme digestion, 10 amplified PCR products in rye but neither in wheat nor in the 1Ri, 1RiS and 1RiL addition lines, and 16 amplified PCR products neither in wheat nor in rye. Although the rye EST primers were designed in the orthologous sequences that were different in wheat from those in rye, no polymorphic PCR product was found in many cases between the 1Ri addition and euploid CS lines. This is probably due to the presence of homoeologous wheat sequences (due to the hexaploid nature of CS) that were not registered in the database at the time of the homology search in this study. The reason for the small number of rye-specific EST markers may be attributed to the small number of available rye ESTs (~10,000) in the dbEST, compared to almost 1,000,000 ESTs of wheat, and to the extremely high similarity of the genomic sequences between rye and wheat found in the course of our homology search (unpublished data). The predominance of 1RiL-specific PCR markers found in this study implies that the sequence homology between 1Ri and wheat group-1 chromosomes is lower in the long arms than in the short arms. The primer sets for Sec1 and IAG95 amplified the expected PCR products in the rye, 1Ri and 1RiS lines. These EST-based PCR markers make good landmarks of chromosome 1Ri.
To characterize the breakpoints of the rearranged 1Ri chromosomes we used nine 1Ri-specific PCR markers identified in this study, which were arm-specific and amplified clear PCR products, and two published PCR markers Sec1 and IAG95. These PCR markers amplified clear 1Ri-specific PCR products without enzyme digestion (Table 1). Using these PCR markers, we analyzed the 55 1Ri dissection lines and found that there was no contradiction between the cytological breakpoints and the PCR analyses (Table 3). Two PCR markers Sec1 and IAG95 were localized on the 1Ri satellite as reported previously (Shimizu et al., 1997 and Mago et al., 2002), dividing it into two regions. Only one of the nine primer sets characterized the 1RiS arm excluding the satellite (Fig. 2). These three PCR markers, together with the secondary constriction, grouped the 1RiS breakpoints into five regions. The remaining eight PCR markers distinguished the 1RiL breakpoints, which were, consequently, grouped into eight regions. Fig. 3 schematically shows the distribution of the breakpoints of the rearranged 1Ri chromosomes.
 View Details | Table 3 PCR analysis of the 1R dissection lines |
 View Details | Fig. 2 Amplification of the PCR markers in the 1Ri dissection lines. A. 1RiS-specific marker TOP1017 (arrows show the rye specific band). B. 1RiL-specific marker KUR1001. |
 View Details | Fig. 3 Schematic representation of the breakpoints of the rearranged 1Ri chromosomes separated by the PCR markers. The underlined numbers in red are the PCR markers and the hyphenated numbers in black or blue are the rearranged 1Ri chromosomes whose breakpoints are indicated with the horizontal lines. The rearranged 1Ri chromosomes with single asterisks are translocations, and the others are deletions. The rearranged 1Ri chromosomes on the right (e.g. 1Ri-34) retained the 1Ri segments below the breakpoints on the diagram and the left ones (e.g. 1Ri-56) retained those above the breakpoints. The leftmost three rearranged 1Ri chromosomes in blue had the 1Ri interstitial segments represented in blue vertical lines. |
As shown in Table 2, most of the 1R dissection lines (46 out of 55) were hemizygous for the rearranged 1Ri chromosomes. It would require much effort to make all dissection lines homozygous, especially for the translocation chromosomes between 1R and wheat chromosomes because homozygous lines would lose parts of wheat chromosomes involved in the translocations. Furthermore, as a rule of thumb, unpaired univalent chromosomes are not distributed to all progeny and often suffer from centromere misdivision in meiosis. Therefore, in use, screening for plants carrying the critical rearranged 1Ri chromosomes is necessary in the progeny of hemizygous dissection lines. Instead of cumbersome cytological screening, we may conduct PCR-based screening using appropriate PCR markers shown in Table 3. The rye genome-specific RAPD marker pSc20H, which is dispersed throughout the rye genome except at telomeric regions and nucleolar organizing regions (Ko et al., 2002), is a universal PCR-based marker for identifying the presence of miscellaneous rye chromatins introduced into common wheat (Katto et al., 2004). This marker also identified the presence of the rearranged 1Ri chromosomes in all the 1Ri dissection lines (data not shown). However, this marker can not be used safely for the PCR-based screening of all 1Ri dissection lines because pSc20H would be amplified in plants carrying the intact 1RiS telosome or 1RiL telosome derived from the rearranged 1Ri chromosomes with the rye centromere by centromere misdivision. For the screening of such rearranged 1Ri chromosomes, we need 1RiS- or 1RiL-specific PCR markers that are located proximal to the breakpoints. For example, in selecting critical plants in the selfed progeny of the hemizygous 1Ri-33 line, first we examined 15 plants and found eight plants carrying the rye-specific marker pSc20H (Fig. 4A). Next, using IAG95, TOP1017 and KUR1001 markers, we analyzed these eight plants and found that all plants had KUR1001 and lacked IAG95 markers but that one of them lacked the TOP1017 marker (Fig. 4B). This result indicated that in self-fertilization, the 1Ri-33 univalent was transmitted to seven plants in the intact form and to one plant in the form of 1RiL telosome generated by centromere misdivision. FISH/GISH analysis confirmed the PCR results and furthermore found that four of the seven plants were homozygous for 1Ri-33. In practice, PCR using TOP1017 is enough to screen for plants useful in chromosome mapping. Thus, PCR screening was demonstrated to be an efficient method of screening for rearranged 1Ri chromosomes in common wheat. At present we can identify the presence of the rearranged 1Ri chromosomes (or chromosome arms) by PCR analysis for most of the dissection lines (48 out of 55) except for the lines whose rearranged 1Ri chromosomes had breakpoints very close to the centromere.
 View Details | Fig. 4 PCR-based screening for plants carrying a critical rearranged 1Ri chromosome. A. PCR amplification with pSc20H in the selfed progeny of a plant of 1R dissection line 1Ri-33. B. PCR amplification with IAG95 (lanes 1, 4, 7 and 10), with TOP1017 (2, 5, 8 and 11), and with KUR1001 (3, 6, 9 and 12). The bands surrounded by the white circles are the rye-specific PCR products for each of the markers. Note 1Ri-33-1-5 had KUR1001 markers but lacked the TOP1017 marker as well as the IAG95 marker, which indicated this plant carried the 1RiL telosome. |
This study was supported in part by Green Technology Project DM-1305, the Ministry of Agriculture, Forestry and Fisheries of Japan. This article is a contribution (No. 597) from the Laboratory of Plant Genetics, Graduate School of Agriculture, Kyoto University.
|