| Edited by Hirokazu Inoue. Niji Ohta: Corresponding author. E-mail: niji@molbiol.saitama-u.ac.jp |
The Calvin-Benson-Bassham cycle is the major metabolic pathway of CO2 assimilation in autotrophic organisms (Calvin, 1956; Macdonald and Buchanan, 1990). Many enzymes are involved in this pathway. Among these enzymes, ribulose-1,5-bisphosphate carboxylase / oxygenase (RuBisCO) is a key enzyme that catalyzes the addition of CO2 to ribulose 1,5-bisphosphate (RuBP) and produces two molecules of 3-phosphoglycerate (3-PGA). This enzyme is localized in the stroma of plastids and consists of one octamer of the large subunit (encoded by the rbcL gene) and one octamer of the small subunit (encoded by the rbcS gene) in plants.
The rbcL gene is located in the plastid genome both in algae and land plants. In rhodophytes, glaucophytes, cryptophytes and some species of heterokonts, the rbcS gene is also located in the plastid genome and constitutes an operon with rbcL, while it is nuclear-encoded in chlorophyta.
RuBisCO genes in plants and algae are classified into two types; cyanobacterial type or β-proteobacterial type, according to phylogenetic analysis of their amino acid sequences deduced from rbcL (Delwiche and Palmer, 1996) and rbcS (Ohta et al., 1997). Chlorophytes and glaucophytes have the former type of RuBisCO genes, while rhodophytes, and cryptophytes and some species of heterokonts originating from secondary-endosymbiosis of rhodophytes to prechromists contain the latter type of RuBisCO genes.
In red algae, heterokonts and cryptophytes, rbcS and rbcL constitute an operon and seem to have coevolved with cbbX. The cbbX gene (formerly cfxQ, renamed cbbX by Stöbe et al., 1998) is located downstream of rbc genes in red algae and cryptophytes (Reith and Munholland, 1995; Ohta et al., 1997; Douglas and Penny, 1999). CbbX is predicted to be involved in RuBisCO expression (Maier et al., 2000) presumably a transcriptional regulator of RuBisCO in red-line algae (Grzebyk et al., 2003). It has been shown that the cbbX insertional mutant of a Rhodobacter sphaeroides suffers impaired growth under photoautotrophic conditions (Gibson and Tabita, 1997).
Both CbbX proteins possess a helix-turn-helix (HTH) motif, Walker ATP-binding motifs (A-GXXGTGKT, B-VLFIDE) and belongs to the AAA+ (ATPases associated with different cellular activities) superfamily as registered in KEGG (Kyoto Encyclopedia of Genes and Genomes). Walker ATP-binding motifs play a protein activating role in many cellular processes (Neuwald et al., 1999), such as protein export (Akiyama et al., 1994), the circadian clock (Hayashi et al., 2004) and regulation of nitrogen fixation in diazotrophic bacteria (Ray et al., 2002).
Cyanidioschyzon merolae, a unicellular red alga, contains the set of genes, rbcL-rbcS-cbbX, in the plastid genome that originated through horizontal gene transfer from β-proteobacteria (Delwiche et al., 1996; Ohta et al., 1997). Interestingly, a cbbX homologue is also found in the cell nucleus by complete genome sequencing of C. merolae (Matsuzaki et al., 2004). In this paper, we use the names cbbX (nuc) to indicate the nuclear cbbX gene, and cbbX (pt) to indicate the plastid cbbX gene in order to clearly distinguish the two C. merolae cbbX genes. In this report, transcriptional analysis of the two cbbX genes was carried out, and a phylogenetic tree inferred from cbbX genes was constructed. We discuss the phylogenetical evolution of cbbX genes and the relationship between the RuBisCO genes and cbbX genes in C. merolae.
Cells of C. merolae strain 10D were grown in the medium of Allen (1959) at 38°C under continuous light as previously described (Suzuki et al., 1992). For the shift up or down experiment of CO2 supply, C. merolae cells were grown at 38°C under continuous light for 24 h bubbled with air containing 0.04% (v/v) CO2, and then shifted up to 5%. For the shift down experiment, they were grown at 38°C under continuous light for 24 h bubbled with air containing 5% (v/v) CO2 and then shifted down to 0.04%. After shift up or down, they were collected chronologically and used following experiments.
The cells were frozen in liquid nitrogen and disrupted by grinding. Total RNA was isolated with RNeasy Plant Mini Kit (QIAGEN, CA, USA), following the instruction manual. The mRNA was isolated using an Oligo (dT)-Cellulose column (Amersham Pharmacia Biotec, NJ, USA). Synthesis and molecular cloning of the cDNA were performed using a lambdaZAP-cDNA synthesis kit (Stratagene, CA, USA). Approximately 10,000 ampicillin resistant colonies were isolated, and plasmids containing cDNA fragments were further cloned in pBluescriptII SK+ using E. coli XL1 Blue as the host strain. Nucleotide sequences of the cDNA were analyzed by the chain termination method with the Taq Dye Terminator Sequencing Kit (Applied Biosystems, CA, USA).
Similarity searches of the putative open reading frames of cbbXs against the GenBank and EMBL databases were performed with the BLAST program (Karlin and Altschul, 1990) at the Genome Net WWW Server (http://www.genome.ad.jp/). Protein motif searches were performed with MOTIF Search at Genome Net WWW Server (http://www.genome.ad.jp/) using default parameters.
Alignments of homologous sequences were made with the Clustal X program (Thompson et al., 1997) using default parameter sets. A phylogenetic tree based on the amino acid sequences deduced from the nucleotide sequences of the cbbX genes was constructed by the maximum likelihood method with the TREE-PUZZLE program for the Power Mac (Strimmer and von Haeseler, 1996) using the JTT model of sequence evolution (Jones et al., 1992) and 1,000 puzzling steps. All the sequences that showed high similarity to CbbX were used to construct the tree (Table 1). Putative transit peptide sequences in nuclear- or nucleomorph- encoded CbbX were excluded to align sequences.
 View Details | Table 1 Bacterial and algal strains used in the phylogenetc tree |
Total RNA from C. merolae cells was collected at the indicated times under continuous light condition. Aliquots (5 μg) of total RNA were separated by electrophoresis and transferred to HybondTM-N+ membrane filters (Amersham Biosciences) using 10XSSC (1.5 M NaCl and 150 mM sodium citrate). The membrane was baked at 80°C for 2 h. Pre-hybridization and hybridization were performed at 70°C in hybridization solution (5XSSC, 50% (v/v) formamide, 7% (w/v) SDS, 0.1% (w/v) N-lauroylsarcosin, 50 mM KH2PO4, 50 mM K2HPO4, 2.5% blocking stock solution) for 3 h and overnight, respectively. After hybridization, the membrane was washed twice with 2XSSC, 0.1% (w/v) SDS at room temperature for 5 min each, and then twice with 1XSSC, 0.1% (w/v) SDS at 70°C for 15 min. Each signal corresponding to objective genes was detected with digoxigenin (DIG)-labeled DNA probes. A part of nuclear-encoded cbbX was amplified by PCR using the forward and reverse primer pair 5’-atgttgggttttacgactagtct-3’ / 5’-gtcgtcagggatactcatcagt-3’ under the condition of 30 cycles of amplification (94°C denaturation, 30 s; 55°C annealing, 1 min; 72°C polymerization, 2 min). The fragment was cloned into the pCR-TOPO cloning vector of TOPO TA Cloning kits (Invitrogen, CA, USA), and the resultant plasmid was used as a template for synthesizing a nuclear-encoded cbbX specific probe with a DIG-PCR labeling kit (Roche), according to the manual by the supplier. DIG-labeled probe was made by PCR by 30 cycles of amplification (94°C denaturation, 30 s; 55°C annealing, 1 min; 72°C polymerization, 2 min) using forward and reverse primers that were initially used when cloning experiment was performed. Plastid-encoded cbbX specific and rbcL specific probes were prepared by the same procedures as the nuclear-encoded cbbX specific probe using the forward and reverse primer pairs 5’-ctgaaggattgctaatgattggt-3’ / 5’-ttattgaaacaaacgactttttaaaatatctt-3’ and 5’-atggctcaatccgtacaagaacgta-3’ / 5’-acttcgccagatgctgcggctgctct-3’, respectively. DIG was detected by using a DIG detection kit (Roche) according to the manual by the supplier. The lengths of detected RNA signals were estimated by calibrating Northern signals with 16S rRNA (plastid encoded 16S ribosomal RNA, 1425 nt), 18S rRNA (nuclear encoded 18S ribosomal RNA, 1789 nt), 23S rRNA (plastid encoded 23S ribosomal RNA, 2853 nt), 28S rRNA (nuclear encoded 28S ribosomal RNA, 3419 nt). The sizes of the rRNAs were checked from C. merolae genome project (http://merolae.biol.s.u-tokyo.ac.jp).
To target and function in the plastid, CbbX (nuc) should have a plastid-targeting sequence followed by the part of functional protein. Approximate 400 clones in the cDNA library were randomly sequenced both from 5’ and 3’ ends. Six clones contained cbbX (nuc) sequence, and 5 of them contained putative transcription initiation site. The site was 95 bp upstream of the first ATG suggesting the translation start site. The region following the second ATG was highly conserved in two CbbXs (Fig. 1). We therefore regarded the region between the first and second ATG in cbbX (nuc) as the plastid-targeting sequence. We also confirmed above plastid-targeting sequence with Target P 1.1 server program (Emanuelsson et al., 2007) that the sequence targeted to the plastid. The number of cbbX clones obtained in the library was unexpectedly high probably because C. merolae was grown in high CO2 concentration with continuous light, and the genes concerning to CO2 assimilation were highly transcribed.
 View Details | Fig. 1 Alignment of amino acid sequences deduced from the cbbX genes encoded by the nuclear and plastid genomes of C. merolae. Amino acid residues shared by both CbbXs are asterisks. Lines indicate the Walker-A and -B ATP-binding motifs. A box indicates a calcium-binding site (EF-hand). Gaps introduced to improve the alignment are indicated by dashes. |
Fig. 1 shows the amino acid sequence alignment of nuclear-encoded CbbX (nuc) and plastid-encoded CbbX (pt) deduced from nucleotide sequences. CbbX (nuc) possesses a putative signal sequence of 94 amino acids targeting the plastid as described above. The identity of the two sequences (excluding the signal sequence) was 50.9%. Both CbbX (nuc) and CbbX (pt) possess a helix-turn-helix (HTH) motif as well as a putative Walker ATP-binding motifs and belong to the AAA+ superfamily (Neuwald et al., 1999). Most AAA+ superfamily proteins form complexes in an ATP-dependent manner. In addition to the Walker ATP-binding motifs, CbbX (nuc) possesses a putative EF hand Ca2+-binding site which CbbX (pt) lacks. The function of CbbX (nuc) may thus differ from that of CbbX (pt) in the use of Ca2+.
The cbbX gene is known to be encoded in the genome of organisms that have a proteobacterial type RuBisCO. A phylogenetic tree inferred from the deduced amino acid sequences of CbbX proteins was constructed using SpoVK as the outgroup (Fig. 2), because the amino acid sequences of CbbX showed poor but significant similarity to those of SpoVKs of Bacillus cereus, B. halodurans and B. subtilis. The bacterial sporulation factor SpoVK is transcribed late during sporulation but its function has not yet been elucidated. The cbbX genes were separated into three clusters: cluster I containing plastid-encoded cbbX genes of of rhodophytes and heterokonts, cluster II containing bacterial chromosomal- or plasmid-encoded cbbX genes of proteobacteria and marine cyanobacteria, and cluster III containing nuclear- and nucleomorph-encoded cbbX genes of C. merolae, Guilldia theta and Rhodomonas salina.
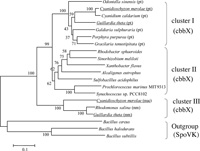 View Details | Fig. 2 Phylogenetic tree inferred from the amino acid sequences deduced from the nucleotide sequence of cbbX. The tree was constructed by the maximum likelihood method with the PUZZLE-TREE software (Strimmer and von Haeseler, 1996). The number at each node shows the reliability value of the branch, which was calculated from 1000 quartet puzzling steps. The scale bar represents 0.1 mutations/site. Branch lengths are drawn to scale. The bacterial and algal strains used in this phylogenetic tree are shown in Table 1. Cluster I is constituted by plastid-encoded encoded CbbXs, cluster II is bacterial chromosome- or plasmid-encoded CbbXs, cluster III is constituted by nuclear- or nucleomorph-encoded CbbXs. sporulation factor SpoVK using as the outgroup. (pt), plastid-encoded; (nm), nucleomorph-encoded; (nuc), nuclear-encoded CbbXs, respectively. Organisms that have two distinct CbbXs are underlined. |
It has been suggested that cryptophytes originated from the secondary-endosymbiosis of prechromist host cells and red algae (Falkowski et al., 2004). The nucleomorph is thought to be the remnant of the nucleus after secondary endosymbiont with ancestral red algae. CbbX (pt) belonged to the same cluster as red algal CbbXs which were encoded in the plastid genome. It is believed that the red algal rbcL-rbcS-cbbX operon originates from horizontal gene transfer of β-proteobacteria (Delwiche and Palmer, 1996), while the other plastid-coding genes originate from ancestral cyanobacteria. The plastid depends more than 90% of its proteins on the product of the cell nucleus, because an ancestral cyanobacterium as the endosymbiont has lost or transferred its genes to the cell nucleus in comparatively early stage of endosymbiosis. This result suggests that after horizontal gene transfer of rbcL-rbcS-cbbX operon from ancestral β-proteobacteria, the copy of cbbX transferred to the cell nucleus. This event might occurr before an ancestral red alga engulfed by ancestral host of a criptomonad alga. Each cbbX encoded in the different organella evolved through different process and clusterIII in Fig. 2 made independent cluster of cluster I and II.
The transcript of cbbX (nuc) was found by sequencing DNA fragments of the cDNA library of C. merolae. Complete nucleotide sequences originate from cbbX transcript existed in the library. Therefore it is suggested that the nuclear-encoded cbbX is not complete pseudogene but actively transcribed.
Fig. 3A shows the results of Northern analysis of the rbcL and cbbX (pt) genes. A 2.8 kb transcript, which corresponded to the length from the start of the rbcL to the end of cbbX (pt) (Fig. 3B), was detected with both rbcL- and cbbX (pt)-specific probes. Upstream of rbcL-rbcS-cbbX (pt) operon, trxM (thioredoxin type m) is encoded on the complementary strand and downstream of the operon, ilvH (acetolactate synthase) is encoded on the complementary strand. Therefore the cbbX (pt) gene was inferred to be cotranscribed with rbcL and rbcS. The rbcL-specific probe also detected a 1.8 kb transcript, which was a rbcL-rbcS transcript.
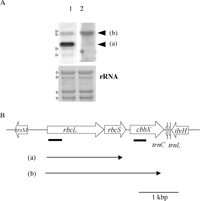 View Details | Fig. 3 Detection of plastid-encoded cbbX. (A) Northern hybridization with the rbcL probe (lane 1) and cbbX (pt) probe (lane 2). The arrowhead (a) and (b) shows rbcL-rbcS and rbcL-rbcS-cbbX transcripts, respectively. Each lane contained 5 μg of total RNA from C. merolae. Methylene blue-stained bands of ribosomal RNA (rRNA) are shown below in order to confirm equal loading of total RNA. Small open arrow heads indicate the positions of rRNAs. (B) Localization of cbbX on the plastid genome. The locations of the transcripts of rbcL-rbcS and rbcL-rbcS-cbbX (pt) are represented as arrow (a) and (b), respectively. trxM = thioredoxin type m, trnC = tRNACys. trnL = tRNALeu and ilvH = acetolactate synthase. Thick bars indicate positions of probes. |
As CbbXs are thought to be involved in CO2 assimilation, we investigated whether CO2 concentration affected the amounts of cbbX transcripts or not. Fig. 4 shows effects of a shift in CO2 supply on the cbbX transcripts. Increasing CO2 concentration from 0.04% to 5% resulted in dramatically increased cbbX (nuc) transcription after 1 h (Fig. 4A, right), whereas the transcription of rbcL-rbcS and rbcL-rbcS-cbbX (pt) was not affected (Fig. 4A, left). Interestingly, reducing CO2 concentration from 5% to 0.04% did not affect in cbbX (nuc) transcription (Fig. 4B, right). These results suggested that cbbX (nuc) responded to changing extracellular CO2 concentration. Transcripts, rbcL-rbcS-cbbX and rbcL-rbcS, changed at the same rate under the changing CO2 growth condition.
 View Details | Fig. 4 Effect of shift up or down of CO2 concentration (v/v) on rbcL-rbcS, rbcL-rbcS-cbbX (pt) and cbbX (nuc) transcription. Northern hybridization analysis was performed under shift of CO2 concentration, 0.04% to 5% (A), and 5% to 0.04% (B), respectively. Hours after shift of CO2 concentration are indicated. In each lane, 5 μg of total RNA from C. merolae was loaded. Methylene blue-stained bands of ribosomal RNA (rRNA) are shown below in order to confirm equal loading of total RNA. Transcripts of rbcL-rbcS, rbcL-rbcS-cbbX (pt) and cbbX (nuc) are indicated by arrows. Small open arrow heads indicate the positions of 28S, 26S, 18S, 16S rRNAs, respectively. |
During the evolution of the red algal linage, cbbX (nuc) may have acquired the role of responding to environmental CO2 concentration, instead of cbbX (pt). The expression of cbbX (nuc) requires stricter regulation than that of cbbX (pt) in the restricted carbon source environment of C. merolae. Plants respond to various stimulations from environments and regulate their genes to survive. C. merolae grows only under photoautotrophic condition, and encodes much smaller number of genes than those of higher plants in its genome. CO2 concentration changes in acidic hot springs in which other organisms live. The gene regulation concerning to Calvin-Benson-Bassham cycle is important in C. merolae.
In a microarray study of the C. merolae plastid genome, the transcription of cbbX (pt) increased under the light condition, although that of rbcL gradually decreased (Minoda et al., 2005). This result suggests that cbbX (pt) responds to light and is functional.
In the future, more nuclear genome sequence of red algae will be analyzed, and very likely reveal the cbbX genes on the nuclear genome of the red algae. As regulation of RuBisCO is hardly clarified regarding transcription, translation and assembly, the study of cbbX (pt) and cbbX (nuc) promises to lead to elucidation of the red alga specific regulation of RuBisCO and of the high specificity of red-type RuBisCO to CO2.
We thank Tsuneyoshi Kuroiwa for his helpful suggestions. This research was supported by Research Institute of innovative Technology of the Earth (RITE) and Grant-in-for Scientific Research to N. O. (No.17570187) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
|