| Edited by Hirokazu Inoue. Niji Ohta: Corresponding author. E-mail: niji@molbiol.saitama-u.ac.jp |
Ribulose 1,5-bisphosphate carboxylase/oxygenase (RuBisCO) is the most abundant and a key enzyme that catalyzes the addition of CO2 to ribulose-1,5-bisphosphate (RuBP), producing two molecules of 3-phosphoglycerate (3-PGA) in Calvin cycle (Ellis, 1979). This enzyme is localized in the stroma of plastids and consists of two octamers of a large subunit (encoded by the rbcL gene) and a small subunit (encoded by the rbcS gene). In chlorophytes, a copy of rbcL is found in the plastid genome, whereas rbcS is in the cell nucleus (Sugiura, 1992). In glaucophytes, a copy of a RuBisCO operon consisting of rbcL and rbcS is located in the plastid genome (Stirewalt et al., 1995). Cyanobacteria also possess a rbcL-rbcS operon (Kaneko et al., 1996).
Cyanidioschyzon merolae, a unicellular rhodophyte, contains in the plastid genome an operon consisting of rbcL, rbcS and cbbX arranged in a tandem array (Ohta et al., 2003). cbbX genes are not found in chlorophytes, glaucophytes and cyanobacteria, but they have been detected in certain proteobacteria. The phylogenetic tree inferred from RbcL indicates that RuBisCO genes of rhodophytes are more closely related to those of β-proteobacteria than those of non-rhodopyte plants or cyanobacteria (Ohta et al., 1997). CbbX contains a nucleotide-binding motif and is presumed to be involved in RuBisCO expression (Maier et al., 2000), possibly in regulation of transcription of RuBisCO operon in red-line algae (Grzebyk et al., 2003). Interestingly, a cbbX homologue is also encoded in the cell nucleus of C. merolae. In this paper, we use the names cbbX (nuc) to indicate the nuclear cbbX gene, and cbbX (pt) to indicate the plastid cbbX gene in order to clearly distinguish the two C. merolae cbbX genes.
The deduced amino acid sequence of CbbX (nuc) possesses a putative transit peptide sequence of 94 amino acids targeting to the plastid (Fujita et al., 2008). The identity of the amino acid sequences of CbbX (nuc) and CbbX (pt) was 50.9% not including the transit peptide sequence. Computer analysis suggested that both CbbX proteins possess a helix-turn-helix (HTH) motif as well as a Ca2+ and/or an ATP binding motif (Fujita et al., 2008).
The RuBisCO operon of C. merolae is transcribed in two ways producing rbcL-rbcS and rbcL-rbcS-cbbX transcripts. There is a strong transcriptional terminator sequence between rbcS and cbbX, which is transcribed when a read through event occurs. Therefore, plastid encoded cbbX transcription depends on the RuBisCO operon promoter and a readthrough.
In this communication, we examine the effect of the above two CbbX proteins on the regulation of RuBisCO operon expression and their role as molecular chaperons of the RuBisCO protein. To perform the experiments, both cbbX genes were cloned and expressed as 6 x His-tagged CbbX proteins to be purified. Binding assays of the CbbXs to RuBisCO promoter DNA and protein-protein interaction assays with CbbXs, RbcL and RbcS were performed. Furthermore, we constructed a plasmid that contained the promoter region of the RuBisCO operon fused to the E. coli lacZ reporter gene. The resulting plasmid was introduced into an E.coli strain, into which a cloned cbbX gene under IPTG inducible promoter was also introduced. The effect of forced expression of the cbbX gene was observed by measuring LacZ activity.
Cells of C. merolae strain 10D were grown under continuous light in the medium of Allen (1959) at 38°C, bubbled with air containing 5% (v/v) CO2 as previously described (Suzuki et al. 1992). For synchronization, the cells were subcultured to < 107 cells/ml and subjected to a 12-h-light/12-h-dark cycle at 38°C (Suzuki et al., 1994).
C. merolae cbbX overexpression plasmids were constructed by insertion of a BspHI-BamHI fragment containing only the coding region of either the cbbX (pt) or the cbbX (nuc) gene into the NcoI and BamHI sites of the expression vector pET-28 (b) (Table 1). The 5’-terminal BspHI and 3’-terminal BamHI sites were introduced into cbbX (pt) and cbbX (nuc) during PCR amplification. The forward primers, complementary to 5’ end regions of noncoding strands of cbbX (pt) and cbbX (nuc), included a BspHI site and an extra NNN site at the 5’ ends. The nucleotide sequences of the forward primers for cbbX (pt) and cbbX (nuc) were 5’ tcatcatgacagtgcaaacatcacaacaga 3’ and 5’ tcatcatgagtatccctgacgacaaagagg 3’, respectively. The reverse primers, complementary to the coding strands of cbbX (nuc) and cbbX (pt) at the 3’ ends, included a BamHI site and an extra NNN at the 5’ ends. The nucleotide sequences of the reverse primers for cbbX (pt) and cbbX (nuc) were 5’ cggcggatcccgttgaaacaaacgactttt 3’ and 5’ cggcggatcccgctccacaatcgcggtctc 3’, respectively. The nucleotide sequences of each insert were confirmed with an ABI Prism 3100 genetic analyzer (Applied Biosystems, CA, USA).
 View Details | Table 1 Strains and plasmids used in this study |
E. coli strain BL21, containing plasmid pET-cbbX (pt) or pET-cbbX (nuc) was grown at 37°C in LB medium to an optical density of 0.5 at 600 nm. The culture was then infused with 1 mM isopropyl-β-D-thiogalactoside (IPTG) and shifted down to 30°C for 2 hours. After induction, the culture was harvested. Cell pellets were resuspended in His-binding buffer (5 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9) and sonicated. Crude cell extracts were cleared by centrifugation (15,000 rpm for 30 min), and loaded onto a Ni+-charge column (His-Bind Resin, Novagen, Darmstadt, Germany) that was equilibrated with column buffer (80 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl pH 7.9). Elution of His-tagged CbbX protein was performed with a buffer containing 1 M imidazole. After dialysis of the sample in a buffer containing 500 mM NaCl and 20 mM Tris-HCl (pH 7.9), the purified proteins were stored at –25°C in the dialysis buffer to which glycerol to a concentration of 25% had been added.
The 3’-digoxigenin (DIG) labeled probe used in the gel mobility shift assays was generated by PCR amplification of fragments containing the putative rbcL promoter region (–177 to +23 relative to the transcription start position). The primer for PCR amplification of the probe was 5’ agaagatctctatctactataaccatctat 3’ and 5’gtacggattgagccatgaatt 3’. The plasmid pTOPO-LSX used as the template for PCR amplification of the rbcL promoter was constructed by ligating pDR2.1-TOPO and promoter DNA amplified by PCR with C. merolae chromosomal DNA and a primer pair (5’ gaagaattcgagtagtgatgagtagttgt 3’ and 5’ agaagatctttattgaaacaaacgactttt 3’). The labeled probe was purified using a QIAquick PCR Purification Kit (QIAGEN, CA, USA) from 2% agarose gel after electrophoretic separation. The probe was resuspended in a buffer containing 10 mM Tris-HCl (pH 8.0) and 1 mM EDTA (pH 8.0).
The protein-DNA binding reaction (15 μl total volume) comprised 5.5 ng of RbcL, DIG-labeled DNA fragment (–177 to +23 relative to the transcription start position), and 1.5 μg of poly (dI::dC) in a buffer of 20 mM Hepes-NaOH (pH 7.6), 1 mM EDTA, 10 mM (NH4)2SO4, 30 mM KCl, 1 mM dithiothreitol and 0.2% Tween-20 (W/V). The reactant was incubated for 1 hour at 20°C. Samples were subjected to nondenaturing gel electrophoresis using 12% polyacrylamide gels in Tris-borate buffer and run at 10 V/cm, then transferred to HybondTM-N+ membrane filters (Amersham Biosciences, Buckinghamshire, UK) using PowerPac 300 (BIO-RAD, CA, USA) at 400 mA for 30 min. Membrane filters were baked at 120°C for 30 min. The detection of DIG-labeled DNA fragments on the membranes was carried out as previously described (Matsumoto et al., 2005).
Total RNAs were isolated from synchronized cells of C. merolae 2 h before end of light, just after onset of darkness, and after 2 h in the dark. The primer extension experiment was carried out as described elsewhere (Matsumoto et al., 2005) with 3’ DIG labeled primer 5’ gtacggattgagccatgaatt 3’, reverse transcriptase (Invitrogen Super ScriptTM II RNase H– Reverse Transcriptase, CA, USA) and RNA to determine the transcription start sites.
Yeast two-hybrid analysis was performed as described before (Yoshimura et al., 2004). Plasmid vectors pGBTK and pGAD424 carrying cloned C. merolae genes were used to transform mating type yeast strains PJ69-4a and PJ69-4α, respectively. The rbcL, rbcS, cbbX (pt) and nuclear-encoded cbbX (nuc, not including the transit peptide sequence) genes were cloned into pGAD424 or pGBTK by inserting PCR synthesized DNA fragments. The cbbX (pt) gene was cloned into pGBTK. The 5’- and 3’-terminal portions of plastid-encoded cbbX were cloned into pGAD424. PCR was performed with template DNA from C. merolae 10D and the primer pairs, 5’ gaattcatggctcaatccgtacaa 3’ / 5’ ctgcagttatacgttagctgttgg 3’ for rbcL, 5’ gaattcatgagagtaacgcaaggt 3’ / 5’ ctgcagttaatatcttgaaccttc 3’ for rbcS, 5’ gaattcatgtcagtgcaaacatca 3’ / 5’ ctgcagttattgaaacaaacgact 3’ for cbbX (pt), 5’ gaattcatgtcagtgcaaacatca 3’ / 5’ ctgctgcagttggtttttggtgctgtatgt 3’ for the cbbX (pt) N-terminal portion, 5’ gaagaattcacatacagcaccaaaaaccaa 3’ / 5’ ctgcagttattgaaacaaacgact 3’ for the cbbX (pt) C-terminal portion, 5’ gaattcatgttgggttttacgact 3’ / 5’ ctgcagttactccacaatcgcggt 3’ for cbbX (nuc) and 5’ gaagaattcatgagtatccctgacgacaaa 3’ / 5’ ctgcagttactccacaatcgcggt 3’ for cbbX (nuc-sp; with transit peptide sequence). The cloned sequences contain the whole putative coding regions, namely, from the start codon to the stop codon for RbcL (488 aa), RbcS (138 aa), CbbX (pt, 293aa), CbbX (nuc, 307 aa) and CbbX (nuc-sp, 401 aa). The CbbX-N (pt) contained at the N-terminal portion of the 115 aa, and CbbX-C (pt) contained 178 aa at the C-terminal portion in the 293 aa of CbbX (pt).
To create the rbcL promoter fused to the lacZ gene of E.coli, putative rbcL promoter region (nucleotide positions –377 to +23 relative to the transcription start position) was amplified by PCR with a primer pair (5’ gaagaattcgagtagtgatgagtagttgt 3’ and 5’ ggaggatccttcctccagaatttcgcttgt 3’) and C. merolae DNA, digested with EcoRI and BamHI, and inserted into the EcoRI / BamHI digested site of pDL2. The resulting plasmid was named pDL400.
The activity of β-galactosidase was measured as described elsewhere (Matsumoto et al., 2005), with the following modifications. The transformants were cultured in 2 ml of LB medium. 900 μl of cells were centrifuged at 15,000 rpm for 5 min at 20°C and suspended in 700 μl Z-buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, 35% β-mercaptoethanol). After addition of 200 μl o-nitrophenyl-β-D-galactopyranoside (ONPG) and 50 μl of toluene, these samples were vortexed vigorously and incubated at 28°C for 5 min. The reaction was terminated by addition of 500 μl of 1 M Na2CO3, and the optical density was measured at 420 nm to determine β-galactosidase activity.
To investigate the role of two CbbX proteins of C. merolae, plasmids pET-CbbX (pt) and pET-CbbX (nuc) carrying His-tagged recombinant CbbXs were constructed and introduced in E. coli BL21. CbbX proteins were overexpressed in E. coli by IPTG addition and purified by Ni+ column chromatography. Increased amounts of proteins with molecular weights corresponding to the estimated MWs 33.6 kDa and 34.8 kDa of CbbX (pt) and CbbX (nuc), respectively, were found in the sample extracted from cells treated with IPTG (Fig. 1A and 1B, lane 2). As most of the CbbX protein was insoluble (Fig. 1A and 1B, lane 4), His-tagged CbbX (pt) and CbbX (nuc) proteins were purified directly by affinity column chromatography of the supernatant samples (Fig. 1A and 1B, lanes 8–10) and used for the following experiments.
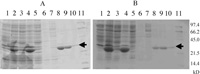 View Details | Fig. 1 Purification of His-tagged recombinant CbbX (pt) and CbbX (nuc) proteins by Ni+ affinity column. (A) CbbX (pt). (B) CbbX (nuc). Lane 1: crude extracts of cells grown without IPTG, lane 2: crude extracts of cells grown with 1mM IPTG, lane 3: supernatant of crude extract of cells grown with 1mM IPTG, lane 4: precipitate of crude extract of cells grown with 1mM IPTG, lane 5: flow through fraction, lanes 6 and 7: washed fractions, lanes 8–10: eluted fraction, lane 11: SDS-PAGE lower marker (BIO RAD). From lanes 5 to 10, the supernatant fraction of crude extract from cells grown with 1 mM IPTG was loaded. The arrows indicate CbbX (pt) and CbbX (nuc). |
In the plastid genome of C. merolae, trxM (thioredoxin type m) is encoded approximate 400 bp upstream of rbcL in the complementary strand (Fig. 2A), and we regarded the rbcL promoter region as in between the coding region of trxM and rbcL. The ability of CbbX (pt) and CbbX (nuc) to bind to the putative rbcL promoter region was analyzed by Gel mobility shift assays (Fig. 2). Two putative DNA-protein complexes of low (L) and high (H) mobility were observed. Since the high mobility complex was observed in the control lane without protein (Fig. 2B, lane 1), we concluded that the high mobility complex was a DNA-DNA complex (probe dimer). Only the low mobility complex was deemed to be a DNA-CbbX complex. As the signal intensity of the CbbX (pt)-DNA complex was higher than that of CbbX (nuc)-DNA, we concluded that while both CbbX (pt) and CbbX (nuc) possess DNA binding activity to the rbcL promoter, the DNA binding activity of CbbX (pt) to the rbcL promoter is higher than that of CbbX (nuc). This may be due to inactive or aggregated CbbX (nuc) proteins formed in the His-tagged sample during purification.
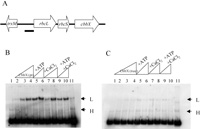 View Details | Fig. 2 Gel mobility shift assay of rbcL promoter DNA by CbbX (pt) or CbbX (nuc). (A) Genetic map of RuBisCO operon and the flanking region. The thick bar indicates the rbcL promoter used for the binding assay. (B), (C) Binding assay of CbbX (pt; B) and CbbX (nuc; C) to the putative promoter region. Effects of ATP and CaCl2 were also investigated. Lane 1, probe only; lane 2, CbbX 0.066 mg/ml and probe; lane 3, CbbX 0.132 mg/ml and probe; lane 4, CbbX 0.264 mg/ml and probe; lane 5, CbbX 0.132 mg/ml and probe with ATP; lane 6, CbbX 0.264 mg/ml and probe with ATP; lane 7, CbbX 0.132 mg/ml and probe with CaCl2; lane 8, CbbX 0.264 mg/ml and probe with CaCl2; lane 9, CbbX 0.132 mg/ml and probe with ATP and CaCl2; lane 10, CbbX 0.264 mg/ml and probe with ATP and CaCl2; lane 11, CbbX 0.264 mg/ml and probe with 6 ng competitor probe. ATP and CaCl2 were used at a concentration of 1 mM. All reactions contained 3 ng of calf thymus DNA. The arrows indicate low and high mobility complexes. |
As the two CbbXs contain an ATP and/or Ca2+ binding motif, we investigated whether the binding activities of CbbX (pt) and CbbX (nuc) are affected by ATP and/or Ca2+. As shown in Fig. 2C and 2D, lanes 5–10, the addition of 1 mM ATP and/or 1 mM Ca2+ to binding reaction did not stimulate DNA binding activities of CbbX (pt) or CbbX (nuc). The effects of ATP and Ca2+ were not reproducible. However it is considered that some factor(s) causing positive effect of ATP or Ca2+ is missing in the binding assay system, or that these motifs require similar nucleotides or metal ions other than ATP or Ca2+.
To check for direct interactions between RuBisCO proteins and CbbX proteins, we used yeast two-hybrid analysis. We first cloned the rbcL, rbcS, cbbX (pt), cbbX (nuc) and cbbX (nuc-sup; with signal peptide) genes into pGBTK to obtain DNA-binding domain fusions and into pGAD424 for activation domain fusion proteins. As the yeast strain carrying pGAD424-CbbX (pt) showed impaired growth, the 5’ or 3’ portion of plastid-encoded cbbX (pt) were cloned into pGAD424. SigX and YpuN of B. subtilis were used as positive controls (Yoshimura et al., 2004). After mating, interactions between fusion proteins in yeast cells were screened through the expression of the reporter genes HIS3 and/or ADE2. As shown in Fig. 3, a positive interaction was clearly observed between CbbX (pt) and CbbX (nuc), although no interaction was observed between CbbX (pt) or CbbX (nuc) and RbcL or RbcS. Interestingly, no positive interactions were observed between N-terminal CbbX (pt) and CbbX (nuc), or between C-terminal CbbX (pt) and CbbX (nuc). These results suggested that the entire CbbX (pt) protein was necessary for the interaction between CbbX (pt) and CbbX (nuc).
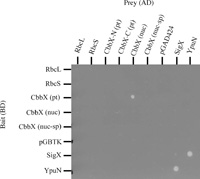 View Details | Fig. 3 Yeast two hybrid analysis of interaction between CbbX (pt) and CbbX (nuc). Diploid strains were constructed by mating PJ69-4a containing pGBTK or its derived plasmids harbouring RbcL, RbcS, CbbX (pt), CbbX (nuc), CbbX (nuc-sp), SigX or YpuN with PJ69-4α containing pGAD424 or its derived plasmids harbouring RbcL, RbcS, N-terminal CbbX (pt), C-terminal CbbX (pt), CbbX (nuc), CbbX (nuc-sp), SigX or YpuN. Two-day-old cells that had been grown on a plate were suspended in culture medium, and 30 μl each of bait and prey plasmid-harboring cells were mixed with 140 μl of media per well, allowing cells to mate during overnight incubation at 30°C. Cells were washed twice in distilled water and resuspended in 20 μl distilled water; in each case a 2 μl aliquot was then spotted onto an SC-LW plate. Plates were incubated at 30°C for 2–3 days; diploid cells were replica plated onto selective SC-LWHA plates containing 5 mM 3-aminotriazole using a replicator and incubated at 30°C for another 5 days. AD, Activation domain; BD, DNA-binding domain. |
The results suggested that CbbX was not a molecular chaperon or a factor directly acting on RbcL and RbcS leading to protein maturation. It was notable that subunits of RuBisCO, RbsL and RbcS did not show direct interaction among subunit proteins. RuBisCO assembly model could explain the above results (Roy, 1989). After RbcL and unknown molecular-chaperon like proteins bind to make the RuBisCO core, eight RbcS subunits futher associate with the core to complete the enzyme (Gutteridge and Gatenby, 1995).
The transcription start site of the operon was determined by the primer extension method (Fig. 4). It has previously been reported that C. merolae cells can be synchronized by subjecting them to a 12 h light/dark cycle (Suzuki et al., 1994). In order to find out whether the light conditions affect the transcription pattern in synchronously growing cells, the growth of C. merolae cells was synchronized and RNAs were extracted at three points of the cycle: light, turning point of light to dark, and dark.
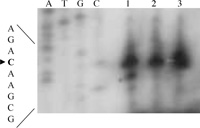 View Details | Fig. 4 5’-end primer-extension analysis of RuBisCO operon mRNA of C.merolae. Lane 1; light 10 h, lane 2; light 12 h/ dark 0 h, lane 3; dark 2 h, respectively. The transcription start site (a C residue) is indicated by an arrowhead. For comparison, sequences were generating using the same primer that was used for the primer-extension. |
Mapping of the 5’-end of the rbcL transcripts were performed by means of a primer extension experiment. The transcription start sites were identical under all three conditions. As shown in Fig. 4, the transcription start site was a C residue, located 23 bases upstream of the translational initiation codon ATG of rbcL. The putative -35 (AGAACA) and -10 (TAAAGT) sequences of the rbcL promoter (Fig. 5A) were very similar to those recognized by plastid-encoded RNA polymerase (PEP, Hajdukiewicz et al., 1997; Hedtke et al., 1997). Transcriptions of photosynthetic genes are regulated by PEP, and are much elevated in the light. On the other hand, constantly expressing housekeeping genes are transcribed by NEP (nuclear-encoded RNA polymerase, Hajdukiewicz et al., 1997; Berg et al., 2004). The region shown in Fig. 5A contains four direct repeats; DR a (ctataaccat), DR b (gtcaa) and DR c (tattcttat, triple repeat) upstream of the transcription start site. DR d (aattc) is between transcription start site and translation start site. Direct repeats of nucleotide sequences are likely candidates for promoter regions at which transcription regulator proteins bind (Tropel and van der Meer, 2008). CbbXs may directly bind to the putative promoter region and repress the transcription of RuBisCO operon, although we did not perform localization of CbbX binding region by DNase I-digestion of CbbX-promoter DNA complex. The distance between the transcription start site and the translation start site of rbcL is much shorter in organisms that constitute RubisCO operon as rbcL-rbcS-cbbX (Fig. 5B, C. merolae and R. eutropha H16) than those constitute only of rbcL-rbcS (Fig. 5B, A. thaliana and Anabaena 7120).
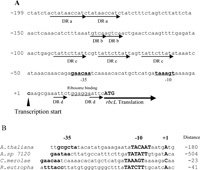 View Details | Fig. 5 Nucleotide sequence of the upstream of translation start site of rbcL in the plastid genome of C.merolae and comparison of the upstream of transcription start site in various organisms. (A) Upstream of translation start site of rbcL. The closed arrow indicates the transcription start site of RuBisCO operon as the position +1, and numbers upstream from this site are shown in the left side (bp). Putative -35 and -10 regions are indicated by bold letters and putative RNA binding site is underlined. The RbcL translation initiation codon (ATG) is shown in capital letters. Direct repeats, DR a (ctataaccat), DR b (gttca) DR c (tattcttat, tripret repeat) upstream of transcription initiation site and DR d (aattc) are indicated by arrows. (B) Comparison of the nucleotide sequences upstream of transcription start site of rbcL in various organisms. A. thaliana (Arabidopsis thaliana, Isono et al., 1997), A. sp 7120 (Anabeana sp. PCC7120, Ramasubramanian et al., 1994), C. merolae (Cyanidioschyzon merolae 10D, Ohta et al., 1997) and R. eutropha (Ralstonia eutropha H16, Bowien et al., 2002). Bold letters indicate putative promoter regions of -35 and -10 sequences, and the transcription start site (+1). Numbers shown in the right of the nucleotide sequence are the distance (bp) from rbcL translation initiation codon. |
Since both CbbXs was found to bind the promoter region of rbcL, we decided to examine the effect of cbbX overexpression on PrbcL-lacZ expression, as a means to assess the effect of the CbbXs on RuBisCO operon expression. When E. coli was transformed by a vector plasmid pDL2 with lacZ alone, it showed a low activity level even when IPTG was added (ca. 250 MU, Fig. 6, first and second bar). When rbcL promoter DNA was inserted upstream of the lacZ gene (PrbcL-lacZ; pDL400), high lacZ activity was observed (ca. 2000 MU, Fig. 6, 3rd and 4th bar). This indicates that the rbcL promoter of C. melorae was recognized in E. coli and lacZ was transcribed by E.coli RNA polymerase.
 View Details | Fig. 6 Effect of CbbX expression on C. merolae rbcL promoter activity in E.coli. E. coli cells were transformed by Promoter-lacZ plasmid pDL2 or pDL400, without or with rbcL promoter region, respectively. pET-28(b), pET-CbbX (pt) and pET-CbbX (nuc) were introduced into above E. coli cells. The optical density was measured to determin LacZ activity. Lane 1, pDL-lacZ; Lane 2, pDL-lacZ plus IPTG; Lane 3, pDL-PrbcL-lacZ; Lane 4, pDL-PrbcL-lacZ and IPTG; Lane 5, pDL-PrbcL-lacZ and pET; Lane 6, pDL-PrbcL-lacZ, pET and IPTG; Lane 7, pDL-PrbcL-lacZ and pET-CbbX (pt); Lane 8, pET-PrbcL-lacZ, pET-CbbX (pt) and IPTG; Lane 9, pDL-PrbcL-lacZ and pET-CbbX (nuc); Lane 10, pDL-PrbcL-lacZ, pET-CbbX (nuc) and IPTG. Data are shown as mean values with the standard deviations of five independent measurements. |
Introduction of the pET-28 (b) plasmid into the above E.coli strain reduced lacZ expression regardless of IPTG addition (Fig. 6, 5th and 6th bar) due to incompatibility as both plasmids, pDL and pET, harbor same replication origin (Thomas et al., 2005). Introduction of pET-28 (b)-cbbX (nuc) or pET-28 (b)-cbbX (pt) did not reduce the LacZ activity further (Fig. 6, 7th and 9th bars, respectively). On the other hand, expression of either cbbX by IPTG addition strongly reduced LacZ expression (Fig. 6, 8th and 10th bars, ca. 200 MU, respectively). In vitro DNA binding ability of CbbX (nuc) was weaker than that of CbbX (pt), however, in vivo inhibitory activity of CbbX (nuc) was similar to that of CbbX (pt). The results suggest that some factor(s) enhancing the DNA binding activity of CbbX (nuc) in vivo in E.coli cell. However, this enhancement may not be observed in C. merolae cell.
Summarizing the above results obtained from in vitro experiments, and in vivo experiments with E. coli cells, it is possible that the RuBisCO operon of C. merolae is negatively regulated by the CbbX (pt) repressor, but not by CbbX (nuc) due to weak DNA binding activity, and that CbbX (nuc) functions as a modifier of CbbX (pt) repressor because of direct interaction of CbbX (nuc) and CbbX (pt) revealed by yeast two hybrid analysis. However, in C. merolae the expression of CbbX (nuc) is enhanced by increased concentration of CO2 (5%) while expression of the RuBisCO operon is not affected by CO2 concentration (Fujita et al., 2008) suggesting that the increased amounts of CbbX (nuc) did not affect the transcription of RuBisCO operon. If CbbX (nuc) functions as a modifier of CbbX (pt) repressor in vivo in C. merolae, increased CO2 concentration should affect expression of the RuBisCO operon on the plastid genome by modifying of CbbX (pt) repressor function. In order to clarify the role of the CbbX (nuc), we are carrying out Northern blot analysis of the effect of higher CO2 concentration, CO2 and light combination on the expression of the cbbX (nuc) and the RuBisCO operon.
This research was supported by the Research Institute of Innovative Technology of the Earth (RITE) and Grant-in-for Scientific Research to N. O. (No.17570187) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
|