| Edited by Koji Murai. Masao Watanabe: Corresponding author. E-mail: nabe@ige.tohoku.ac.jp |
In higher plants, plastids are a major site of biosynthesis of fatty acid, which are major constituents of every membrane in cells and are also found outside of cells in cuticular lipids. The de novo fatty acid biosynthesis pathway is one of the most important primary metabolic pathways in all cellular organisms, generating the palmitic and stearic acids that serve as the precursors for other fatty acid of different lengths and saturation levels. In plant cells, most fatty acid are found in lipid forms, which function either as essential components of cell membranes or as important regulators of cell growth and differentiation (Ohlrogge and Browse, 1995; Topfer et al., 1995). Therefore, a null mutation that completely blocks de novo fatty acid biosynthesis would almost certainly be lethal.
The de novo biosynthesis of fatty acid is performed by fatty acid synthase (FAS), with acetyl-CoA used as the starting unit and malonyl-acyl carrier protein (ACP) as the elongator. Unlike animal FAS, which is a multifunctional protein, plant FAS is an easily dissociable multisubunit complex consisting of multiple monofunctional enzymes, including 3-ketoacyl-ACP synthase (KAS), 3-ketoacyl-ACP reductase, 3-hydroxyacyl-ACP dehydrase, and enoyl-ACP reductase (Ohlrogge and Jaworski, 1997), whose primary products are palmitic acid (16:0) and stearic acid (18:0). These 16:0 and 18:0 fatty acid subsequently enter multiple lipid metabolic pathways to form various glycerolipids and phospholipids, such as diacylglycerol, triacylglycerol, sphingomyelin, and ceramide, to yield very long chain fatty acid, such as cuticular waxes; or to be converted into phytohormones, such as jasmonic acid (Ohlrogge and Browse, 1995).
The 3-ketoacyl-ACP synthase (KAS) enzyme creates new carbon-carbon bonds by carrying out Claisen condensation which consists of three steps: (i) transfer of an acyl primer substrate from acyl carrier protein (ACP) or CoA to a cystein residue of the condensing enzyme; (ii) decarboxylation of an ACP-bound elongating substrate to give a carbanion; and (iii) condensation of the carbanion with the carbonyl carbon of the enzyme-bound acyl primer. Three KAS enzymes participate in synthesis of the fatty acid carbon skeletons in plant plastids and Escherichia coli as part of multifunctional fatty acid synthase complexes in which each protein is encoded by a discrete gene. KAS III is involved in the first condensation of acyl-CoA and malonyl-CoA. KAS I is involved in the elongation of the carbon chain up to 16 carbons, and KAS II is involved in the last condensation steps from 14 and 16 carbons to 18 carbons (Harwood, 1996).
The fatty acid biosynthesis 1-1 (fab1-1) mutant of Arabidopsis is partially deficient in activity of AtKAS2, and its mutation is a single nucleotide change in AtKAS2 that results in a Leu337Phe substitution (Carlsson et al., 2002). In the fab1-1 mutant, the biochemical defect appears to be a reduction in the activity of the AtKAS2 responsible for the elongation of palmitic acid (16:0) to stearic acid (18:0). The fab1-1 mutant revealed a high content of palmitic acid in leaves, roots and seeds (Wu et al., 1994) and a sensitivity to low temperatures, which leads to damage and death of fab1-1 plants (Wu et al., 1997). Because the fab1-1 mutant appears to be leaky, the changes in overall membrane fatty acid composition are moderately small. In this study, in order to determine the developmental function of the AtKAS2 gene in detail, we performed phylogenetic and expression analyses, and chose a reverse genetic approach with a severe mutant line (a T-DNA-disrupted atkas2 mutant). The AtKAS2 gene was expressed in rosette leaves, stems, flower buds, open flowers, and highly in siliques. No homozygous atkas2 mutant plants were identified in the segregation analysis, indicating that disruption of the AtKAS2 gene causes embryo lethality. Arrest of embryogenesis occurred predominantly at globular stages and affected both embryo pattern formation and normal endosperm development.
Arabidopsis ecotype Columbia-0 (Col-0) plants were grown in a SANYO growth chamber (MLR-1546) at a mean temperature of 23°C under a 16-h-light/8-h-dark photoperiod. The atkas2 mutant line was obtained from the Columbia ecotype line SALK_018337, generously donated by Joe Ecker (Salk Institute, La Jolla, CA) to the Arabidopsis Biological Resource Center (ABRC). One fully expanded leaf of a 4-week-old plant was used to extract genomic DNA. To determine plant genotypes, AtKAS2 gene-specific primers (G1: 5’-TTCTGGATCCGCTAGCATTC-3’ and G2: 5’-CAGTGTTTCTAGCATCCACC-3’) and the left border of T-DNA primer (LBa1: 5’-TGGTTCACGTAGTGGGCCATCG-3’) were used in PCR using genomic DNA as template. One hundred ng of genomic DNA was used for genomic PCR. PCR was performed with TaKaRa Ex Taq DNA polymerase (TaKaRa Shuzo) for 40 cycles of denaturation for 30 seconds at 94°C, annealing for 30 seconds at 55°C and extension for 2 minutes at 72°C. Transmission of mutant alleles was determined through reciprocal testcrosses of heterozygous atkas2 and wild-type Col-0 plants. Outcrosses were performed by applying pollen from newly dehiscing flowers onto the stigmas of flowers that had been surgically emasculated just prior to dehiscence.
Sequence alignment was performed with Clustal W (Thompson et al., 1994) and phylogenic trees with the Tree View program (Zhai et al., 2002) using the Neighbor-Joining method (Saitou and Nei, 1987) as described in Hakozaki et al. (2004).
For RT-PCR analysis, mRNA was isolated from rosette leaves, stems, siliques, roots, flower buds and open flowers. Each organ was collected from plants grown in a growth chamber for 5 to 7 weeks. Isolation of mRNA from each tissue was performed using a Fast Track 2.0 mRNA isolation kit (Invitorogen, San Diego, CA, USA) as described in Takada et al. (2001). Genomic DNA was extracted from one fully expanded leaf of a 4-week-old plant. After isolation of mRNA, First-strand cDNA was synthesized from 150 ng of mRNA using a cDNA synthesis kit (Amersham Biosciences UK Limited, Buckinghamshire, UK). After the RT reaction, the cDNA was subjected to PCR reactions. A 150 ng portion of mRNA was used for each RT-PCR, and 100 ng of genomic DNA was used for genomic PCR. PCR was performed with TaKaRa Ex Taq DNA polymerase (TaKaRa Shuzo) for 29 cycles of denaturation for 30 seconds at 94°C, annealing for 30 seconds at 55°C and extension for 30 seconds at 72°C. The following pairs of primers were used: 5’-TCTCTCACGGTGGAGGAGATA-3’ and 5’-GGCTAATACCACTGTTGCCTT-3’ for AtKAS2 (At1g74960), 5’-GAGAAGATGACTCAGATCATG-3’ and 5’-ACCTGCTGGAAAGTGCTGA-3’ for actin 4 (At5g59370).
The 1,584-bp upstream region of AtKAS2, combined with the complete AtKAS2 gene and 495-bp untranslated 3’ region, was cloned into the plasmid pIG101. Transformed Arabidopsis plants carrying the atkas2 allele were selected for hygromycin and kanamycin resistance. One hundred ng of genomic DNA was used for genomic PCR. PCR was performed with TaKaRa Ex Taq DNA polymerase (TaKaRa Shuzo) for 40 cycles of denaturation for 30 seconds at 94°C, annealing for 30 seconds at 55°C and extension for 2 minutes at 72°C. To determine plant genotypes, AtKAS2 gene-specific primers (G3: 5’-GTAATGCTCTGTGGTGGCTCAGATGCA-3’ and G4: 5’-CTACTTATGGCTCTCTTATACAAGGCTGA-3’) and the left border of T-DNA primer (LBa1: 5’-TGGTTCACGTAGTGGGCCATCG-3’) were used in PCR using genomic DNA as template.
The promoter region of AtKAS2 gene was identified from a DNA database of genomic sequences of Arabidopsis. These promoter regions were amplified by PCR using each of the following primers: AtKAS2pro-F (5’-CTCGAGGGTGTTCATGGATAGTGGAT-3’) and AtKAS2pro-R (5’-GGATCCGGCATAAAAAGGAAGAGGCTTTGAG-3’). All PCR fragments were subcloned into the pCR2.1 plasmid vector (Invitrogen). The nucleotide sequence of these promoter fragments was confirmed by sequencing of the plasmid using the DNA sequencer (ABI 310; PE Biosystems, Foster City, CA) as described in Park et al. (2006b). The plasmid pIG121-Hm (Ohta et al., 1990) containing the neomycin phosphotransferase II (nptII) and β-glucuronidase (gus) genes was introduced into the Agrobacterium tumefaciens strains EHA105 (Hood et al., 1993) for transformation. These bacterial strains were maintained on a YEP (5 g/l yeast extract, 5 g/l bactopeptone, 5 g/l sucrose, pH 7.2) agar plate containing 50 mg/ml kanamycin. A single colony was grown overnight in liquid YEP broth at 28°C with appropriate antibiotics, sedimented using a centrifuge (3,000 g), and resuspended in liquid MS basal medium for plant transformation.
Transgenic Arabidopsis plants were generated by the floral-dip method (Clough and Bent, 1998). Transformation was carried out using Agrobacterium tumefaciens strains EHA105 (Hood et al., 1993). Transformed plants were selected on Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) containing 0.8% (w/v) agar, 1% sucrose and 50 mg/L kanamycin. For GUS staining, several tissues were isolated from transgenic plants harboring the AtKAS2 promoter::GUS fusion and then immersed in a solution containing 50 mM sodium phosphate buffer (pH7.0), 10 mM EDTA, 0.5 mM potassium ferrocyanide, 0.5 mM potassium ferricyanide, 0.1% Triton X-100 and 0.5 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-glucuronide. After staining overnight at 37°C, samples were washed three times with 50 mM sodium phosphate buffer and then subjected to microscopic analysis which was carried out according to Park et al. (2006a) and is described below. Vegetative and floral tissues were used in GUS assay. Stained seedlings and tissues were fixed in 50% ethanol, 5% acetic acid, and 3.7% formaldehyde and destained in 70% ethanol overnight. Additional overnight clearing with lactophenol (water:glycerol:lactate:phenol, 1:1:1:2 [v/v]) was performed for seeds after the ethanol treatment to visualize the embryos.
Siliques of different developmental stages from heterozygous AtKAS2/atkas2 plants and wild type were dissected with hypodermic needles. Ovules from individual siliques were mounted on microscope slides in a clearing solution (Liu and Meinke, 1998). Images were obtained using either the Nikon ECLIPSE E800 or SMZ 1500 microscope (http://www.nikon.co.jp).
Three classes of KAS enzyme have been described: Class III is involved in the first condensation of acetyl-CoA and malonyl-CoA, class I in the elongation of the carbon chain up to 16 carbons, and class II is involved in the last condensation steps from 14 and 16 carbons to 18 carbons (Harwood, 1996) (Fig. 1).
 View Details | Fig. 1 Simplified schema of function of KAS families in a generalized plant cell. |
The fab1-1 mutant is partially defective in activity of AtKAS2 gene and, Brassica napus KAS II cDNA complements the fab1 mutant phenotype (Carlsson et al., 2002). In order to compare the plant KAS enzymes and AtKAS2, we performed multiple alignments of plant KAS and generation of a phylogenic tree. The AtKAS2 gene contains 541 amino acid residues with a molecular mass of 57.6 kDa and a pI of 8.1. The AtKAS2 has 94.2% identity with Brassica napus KAS II (accession number AAF61739), 78.9% with the Glycine max KAS II (AAF61737) and 76.6% with the Cuphea wrightii KAS II (AAB37270).
Multiple alignments were performed for the three plant proteins with high similarity to the AtKAS2 protein (Fig. 2A). A functional-domain analysis with Pfam 7.0 (http://www.sanger.ac.uk/Software/Pfam) indicated that the region between the 130th and 375th amino acid residues forms the β-ketoacyl synthase N-terminal domain (e-value: 1.30e-75, box in Fig. 2A), which contains most of the structures involved in dimer formation and also the active site cysteine (grey box in Fig. 2A). Furthermore, the region between the 383rd and 497th amino acid residues forms the β-ketoacyl synthase C-terminal domain (e-value: 2.4e-48, doted box in Fig. 2A). The structure of β-ketoacyl synthase is similar to that of the thiolase family and also to that of chalcone synthase. A phylogenic tree was generated with several plant KAS protein sequences of classes I, II, and III. The tree confirmed that the AtKAS2 belongs to class II (Fig. 2B).
 View Details | Fig. 2 (A) Multiple alignment of AtKAS2 and its related protein sequence from GenBank. Sequences were aligned with the aid of the CLUSTAL W program (Thompson et al. 1994). Sequence accessions are as follows: Cuphea wrightii (AAB37270), Glycine max (AAF61737), Arabidopsis thaliana (AAK69603) and Brassica napus (AAF61739). The grey box indicates the conserved active site cysteine. The asterisk (*) indicates the identical amino acids, and the dot (.) denotes similar amino acids. The box indicates the β-ketoacyl synthase N-terminal domain, and the dotted box indicates the β-ketoacyl synthase C-terminal domain. (B) An unrooted phylogenetic tree of plant protein sequence of KAS proteins. The dendrogram was generated by the neighbor-joining method (Saitou and Nei 1987). AtKAS I, AtKAS II and AtKAS III from Arabidopsis thaliana (Accession No. AAM65396, AAK69603 and AAM14214, respectively). BnKAS II from Brassica napus (Accession No. AAF61739). ChKAS III (Accession No. AAF61398). CpKAS I from Cuphea pulcherrima (Accession No. ABA07910). CwKAS II and CwKAS III from Cuphea wrightii (Accession No. AAB37270 and AAA97533, respectively). GmKAS I, GmKAS II and GmKAS III from Glycine max (Accession No. AAF61730, AAF61737 and AAF70509, respectively). OsKAS I-1, OsKAS I-2, OsKAS II and OsKAS III from Oryza sativa (Accession No. BAF14818, BAF18967, BAF22188, BAF15964, respectively). Scale bar represents amino acid substitutions per site. |
To analyze the expression pattern of AtKAS2, we performed RT-PCR using mRNA from various tissues of wild-type Arabidopsis with gene specific primers in exons 1 and 2. The results, standardized by the constitutive Actin 4 expression, showed that AtKAS2 was expressed in rosette leaves, stems, flower buds and open flowers, highly in siliques, and not in roots (Fig. 3A). To investigate the temporal and spatial expression pattern of the AtKAS2 gene, we performed promoter::GUS analysis. A 1.5-kb fragment containing the AtKAS2 promoter was fused to the β-glucuronidase (GUS)-encoding uidA reporter gene. The AtKAS2 promoter::GUS construct was introduced into wild-type Arabidopsis plants by Agrobacterium infiltration and five T1 transformants were analyzed histochemically for GUS activity. GUS activity was not detected in the globular stage of the embryo (Fig. 3B), while blue signals of GUS activity were observed in the late globular and early heart stage (transition stage of globular to heart) (Fig. 3C), heart stage of the embryo (Fig. 3D), torpedo stage of the embryo (Fig. 3E) and cotyledonary stage of the embryo (Fig. 3F). In 4-day old seedlings, the shoot apex and stomatal guard cells showed GUS expression (Fig. 3G, H), but root and hypocotyl cells did not (Fig. 3G). At the 25-day stage, the inflorescences were stained positive for GUS expression (Fig. 3I). Furthermore, strong GUS activity was also observed in the styles and pollen grains (Fig. 3J, K), but none was detected in the unfertilized ovules (Fig. 3J). Nearly half of pollen grains did not show the GUS signals, owing to a heterozygous state of the promoter::GUS transgene. These results indicated that the high expression of AtKAS2 in siliques detected by RT-PCR corresponds to its expression in developing seeds. Similarly, RT-PCR detection of AtKAS2 expression in the rosette leaves and open flowers might correspond to its expression of the stomata cells and the styles/pollen grains, respectively.
 View Details | Fig. 3 Expression of AtKAS2 in Arabidopsis. (A) RT-PCR analyses of AtKAS2 mRNA levels in various organs. Each organ was collected from plants grown in soil for 5 to 6 weeks. Rl, rosette leaf; St, stem; Si, silique; Ro, root; Fb, flower bud; Of, open flower; Ge, genome DNA. GUS expression patterns of the transgenic plants harboring the AtKAS2 promoter-GUS fusion construct. GUS activity in globular stage embryo (B), late globular and early heart stage embryo (C), heart stage embryo (D), torpedo stage embryo (E), cotyledonary stage embryo (F), 4-day old seedling (G), close-up of leaf stomatal guard cells (H), inflorescence (I), unfertilized and fertilized flowers (J) and anther (K). Bars = 100 μm. |
To investigate possible roles of the AtKAS2 gene (At1g74960) in plant development, we used a T-DNA insertion line, SALK_018337 (heterozygous atkas2 mutant) in the Salk Arabidopsis T-DNA insertion population (Alonso et al., 2003). The T-DNA insertion site was determined by sequencing PCR products amplified from genomic DNA of the line with a combination of gene-specific and T-DNA-specific primers shown in Fig. 4A. The atkas2 contains the T-DNA insertion in the sixth intron 1,922 nucleotides downstream of the start codon of the predicted AtKAS2 gene, indicating that the T-DNA inserted line is expected to have a recessive null mutation of the AtKAS2 gene.
 View Details | Fig. 4 Isolation and characterization of the AtKAS2 T-DNA insertion line. (A) The AtKAS2 3385-bp open reading frame encodes a predicted protein measuring 541 amino acids. The AtKAS2 gene contains 13 exons (open boxes) in the coding region. Salk_018337 line contains a T-DNA insertion in the fifth intron 1922-nucleotides downstream of the start codon of the AtKAS2 (At1g74960) gene. The dotted line indicates outer region of the AtKAS2, which was not included in the AtKAS2 pro::AtKAS2 construct for complementation. Horizontal arrows labeled G1, G2, G3, G4 and LBa1 represent the binding site locations for PCR primers. Note that amplification with a G1–G2 or G3–G4 primer combination is impossible in the case of the atkas2 genome because of too large of a T-DNA insertion. (B) PCR results for genomic DNA and dissected immature siliques of the wild type (AtKAS2/AtKAS2), the heterozygous mutant line (AtKAS2/atkas2), and the complement T0 plant harboring atkas2 mutant allele (AtKAS2/atkas2 with AtKAS2 pro::AtKAS2). The primer combination G1–G2 is a diagnostic for the endogenous gene and the combination LBa1-G1 for the T-DNA insertion. An immature silique from the wild type (AtKAS2/AtKAS2) showed only green seeds, whereas the heterozygous mutant line (AtKAS2/atkas2) contained aborted seeds (arrows). Reduction of aborted seeds (arrow) was observed in the complement T0 plant because the atkas2/atkas2 embryos with AtKAS2 pro::AtKAS2 could be normally developed. (C) PCR analysis showing existence of the normally developed atkas2/atkas2 embryo in the silique of complement T0 plant. Among T1 plants harboring the AtKAS2 pro::AtKAS2 transgene, the atkas2/atkas2 homozygous plants were observed; absence of bands in the lane G3–G4 indicated the atkas2/atkas2 homozygous genotype, and amplification of bands with the LBa1-G1 and G1–G2 primer sets demonstrated the existence of the inserted T-DNA and the exogenous AtKAS2 pro::AtKAS2 gene, respectively. To the contrary, in the wild type (AtKAS2/AtKAS2), PCR fragments were detected in the G1–G2 and G3–G4 lanes and not in the LBa1-G1 lane. |
The heterozygous AtKAS2/atkas2 insertion had no effect on sporophytic development in a normal growth condition (data not shown). To obtain homozygous atkas2/atkas2 mutant plants, the heterozygous plant was allowed to self-pollinate. Despite extensive screening, no homozygous atkas2/atkas2 mutant plants were identified by segregation analysis of progeny derived from AtKAS2/atkas2 heterozygotes (Table 1). The genotype of the progeny plants could be distinguished by PCR amplification with 2 primer sets, G1-LBa1 and G1-G2 (Fig. 4A). The AtKAS2/atkas2 heterozygous lines always yielded seeds segregated for AtKAS2/AtKAS2 and AtKAS2/atkas2 in the ratio of 1:2 (Table 1, χ2 = 0.253, P >> 0.05). Moreover, developmental functions of the male and female gametes were not affected by atkas2 mutation because reciprocal crosses between heterozygous mutants and wild-type plants showed that genetic transmission of the atkas2 mutation through the male and female gametes was normal (data not shown). These results strongly suggested that homozygotes of the recessive atkas2 allele might be lethal. In fact, in the siliques of the AtKAS2/atkas2 heterozygous plants, after the greening stage (i.e., late heart to torpedo stages of embryo development), abnormal shrunken-seeds became readily distinguishable as white seeds compared with normal green seeds (Fig. 4B). We considered that the shrunken-seed phenotype was associated with homozygous atkas2/atkas2 mutation because none of the shrunken seeds but all of the normal seeds were able to germinate (data not shown). To quantify this phenomenon, the number of viable or aborted seeds in siliques of the AtKAS2/atkas2 heterozygous lines was counted (Table 2). The segregation ratio of seeds (normal:abnormal) in the AtKAS2/atkas2 heterozygotes was consistent with 3:1 (Table 2, χ2 = 0.127, P > 0.05) as expected for a recessive mutation in an essential nuclear gene.
 View Details | Table 1 Genetic transmission of atkas2 mutations |
 View Details | Table 2 Segregation analysis of embryo lethality associated with the atkas2 mutation |
Furthermore, to achieve complementation of atkas2 mutant, kanamycin-resistant heterozygous (AtKAS2/atkas2) plants were transformed with AtKAS2 pro::AtKAS2 (A 1.5-kb AtKAS2 promoter plus full length AtKAS2 including the 5’ and 3’ untranslated region, and a hygromycin-resistance gene) construct. Seven transformed plants (T0) which survived under the kanamycin and hygromycin selection were found to be heterozygous (AtKAS2/atkas2) and to possess AtKAS2 pro::AtKAS2. They had normal vegetative growth and produced seeds (T1) with reduced appearance of aborted phenotype (Fig 4B); the genotype of aborted seeds might have been “atkas2/atkas2 without AtKAS2 pro::AtKAS2” whose appearance ratio was calculated to be 6.25% of the T1 progeny if the T0 plant possessed the AtKAS2 pro::AtKAS2 transgene at one locus. To confirm production of complemented T1 plants (normally developed T1 plants of atkas2/atkas2 with AtKAS2 pro::AtKAS2), we performed PCR genotyping of T1 plants with the AtKAS2 pro::AtKAS2 transgene, which were normally developed on the hygromycin-containing medium (Fig. 4A, C; see legend in detail). Existence of the living atkas2/atkas2 homozygous plants with the AtKAS2 pro::AtKAS2 construct indicated that exogenous AtKAS2 gene could functionally complement atkas2 (Fig. 4C). Thus, we concluded that the T-DNA disrupted mutation in AtKAS2 caused the embryo lethal phenotype.
In immature siliques of AtKAS2/atkas2 plants, the atkas2/atkas2 embryos were indistinguishable from other embryos before the onset of visible chlorophyll accumulation at the transition from globular to the early heart stage (Mansfield et al., 1991). At the late heart stage, the abnormal atkas2/atkas2 seeds became distinguishable as white seeds compared with that of green normal seeds. In older mature siliques, the atkas2/atkas2 seeds with arrested development eventually turned brownish and dried out. In order to dissect abnormal embryogenesis of atkas2/atkas2, embryo development was compared by Nomarski microscopy in different developing seeds of siliques growing on the AtKAS2/atkas2 plants. The embryogenesis of normal and mutant embryos viewed with Nomarski optics is shown in order in Fig. 5. At the globular stage, the mutant embryos were indistinguishable from normal ones (Fig. 5A, E). However, the developmental arrest occurred in a quarter of the seeds in the siliques when the embryos were in transition from the globular to the heart stage (Fig. 5B, F). A comparison of normal and mutant embryos at the globular (Fig. 5A, E), heart (Fig. 5B, F), and torpedo (Fig. 5C, G) stages showed that mutants remained as small globular embryos. Despite aberrant embryo development, endosperm development of seeds harboring atkas2/atkas2 embryos appeared normal until the early torpedo stage of normal development (Fig. 5B, C, F, G), after which the endosperm and integument layers also started to disintegrate (Fig. 5D, H).
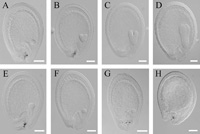 View Details | Fig. 5 Seed development in atkas2 heterozygous. Siliques from heterozygous atkas2 plants were examined at various stages of seed development. (A, E), two representative embryos from the youngest silique, in which all embryos were at the globular stage and indistinguishable; (B, F), older silique with most embryos at the heart stage (B) and with embryos arrested at the globular stage (F); (C) developing early torpedo stage embryo and (G) arrested embryo at the globular stage; (D), oldest silique with developing embryo at the late torpedo stage and (H) globular arrested embryo from the same silique. Bars indicate 50 μm. |
Our alignment and phylogenetic analyses showed that the AtKAS2 (At1g74960) gene encodes a functional protein having KAS II activity, being consistent with the complementation experiment of leaky fab1-1 mutant (Carlsson et al., 2002) and with the recently reported fatty-acid composition of knockout fab1-2 mutant (Pidkowich et al., 2007). Thus, AtKAS2 catalyzes the elongation of 16:0-ACP to 18:0-ACP in plastids, and may have many important biological functions in various tissues in various developmental stages.
The promoter::GUS analysis showed that AtKAS2 is expressed in various specific tissues rather than ubiquitously expressed in all tissues. In particular, the AtKAS2 expression is strong in cotyledonary stage embryos in siliques, suggesting that the pathway regulated by AtKAS2 plays an important role in embryogenesis. Similarly, other fatty acid synthesis genes for Arabidopsis acyl carrier protein (Acl1.2), Brassica napus stearoyl-ACP desaturase, and Arabidopsis acetyl-CoA carboxylase 1 (ACC1) are mainly expressed in developing seeds, and also in several other cell types including flower tissues (Baerson et al., 1994; Baud et al., 2003; Slocombe et al., 1994). Because many EMB genes (whose mutation results in embryo lethality) are not embryo specific in their pattern of expression which is consistent with the requirement for their basal functions throughout the life cycle (Tzafrir et al., 2004), AtKAS2 would be categorized in EMB and fatty acid related genes on the expression profile.
It is noteworthy that AtKAS2 is distinctly expressed in pollen (Fig. 3G). In Oryza sativa, the candidate ortholog of KAS2 was highly expressed in anther compared with leaf (Endo et al., 2004). Although male and female genetic transmission of the atkas2 allele was normal (data not shown) demonstrating that the atkas2 mutation does not critically affect the gametophyte development and function, it is interesting to be aware of the AtKAS2 function in the pollen grains and anthers in connection with importance of fatty acid in the pollen development and fertility (e.g. cer mutants; Ariizumi et al., 2003; Aarts et al., 1995; Millar et al., 1999).
We reported here that disrupted mutation of the AtKAS2 gene causes abnormal embryo development, resulting in embryo lethality. This analysis of the atkas2 mutant raises a new and intriguing question about the possible role of lipids and plastids in plant development. During plant embryogenesis, the zygote undergoes a series of highly orchestrated developmental changes involving cell division, elongation, and differentiation. Seed development is a critical and complex developmental stage in the life cycle of flowering plants, which involves a large number of diverse gene expressions and complex gene regulations (Chaudhury et al., 2001; Goldberg et al., 1994; Meinke, 1995). The heart stage embryo displays both apical-basal polarity and radial symmetry, and is the first stage when the cells that form the meristems of the seedling can be distinguished. These features are not evident in globular-stage embryos, and therefore the transition between the globular and heart stage is a key developmental stage. Many embryo-lethal mutants in Arabidopsis show termination of embryo development during the preglobular to cotyledonary stages (Tzafrir et al., 2004). The largest class of embryo lethal mutants within ethyl methanesulfonate mutagenesis and T-DNA insertional inactivation is that which remains morphologically globular in shape. Therefore, it is considered that the transition from the globular to the heart stage is the most sensitive step of embryo development. The atkas2 mutant belongs to this class, because phenotypic analysis of atkas2 mutant embryo indicated that developmental arrest occurred in the transition globular to heart stage embryo.
The recent studies of embryo lethal mutants caused by disruption of fatty acid synthesis related genes suggested that some fatty acid synthesis related genes are required for embryogenesis. ACC1 encodes an acetyl-CoA carboxylase, which catalyses the carboxylation of acetyl-CoA, forming malonyl-CoA and works in the plastid for fatty acid synthesis. In the acc1 mutant, impaired embryo morphogenesis lacking cucumber-like structures in cotyledons was observed, and triacylglycerides are synthesized at a lower concentration than in the wild-type seed (Baud et al., 2003). Lysophosphatidyl acyltransferase (LPAAT) is a pivotal enzyme controlling the metabolic flow of lysophosphatidic acid into different phosphatidic acids in diverse tissues. Apparently, plastidic LPAAT is essential for embryo development in Arabidopsis during the transition from the globular to the heart stage when chloroplasts begin to form (Kim and Fuang, 2004; Yu et al., 2004). The disruption of the E2 subunit of the plastid pyruvate dehydrogenase complex also causes an early embryo-lethal phenotype (Lin et al., 2003).
KAS II is involved in the condensation steps from 14 and 16 carbons to 18-carbon fatty acid (Harwood, 1996). The 18-carbon fatty acid are required for chloroplast biogenesis via eukaryotic and prokaryotic pathways, and the 18-carbon acyl chains serve as precursors for other vital lipids such as cutin, suberin and waxes. AtKAS2 is needed for the plastid development because chloroplasts of the fab1-1 mutant show abnormal differentiation after chilling treatment (Wu et al., 1997). In addition, it has been known that all plant cells produce fatty acid from acetyl-CoA by a common pathway localized in plastids (Ohlrogge and Jaworski, 1997), and KAS II is a nuclear-encoded protein which localizes and functions in plastids. The fact that embryo development of the atkas2 mutant was arrested just at the transition from globular to the heart stage, when chloroplasts started to develop, suggests that the 18-carbon fatty acid might be indispensable for embryogenesis and chloroplast development.
Because of the lethality in the atkas2 mutant, we were unable to investigate adult plants of atkas2 homozygous mutant. If they are isolated by embryo rescue technique, new insights into the function of AtKAS2 might emerge. Although further experiments are needed to elucidate AtKAS2 function mechanistically, this study demonstrates that AtKAS2 is important for embryogenesis.
This work was supported in part by Grants-in-Aid for Special Research on Priority Areas (Nos. 18075003), a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Rice Genome Project MA-2211) and Grants-in-Aid for the 21st Century Center of Excellence Program from the Japan Society for Promotion of Science (JSPS) to MW, and a grant for the Promotion of Basic Research Activities for Innovative Biosciences from the BRAIN to MKK. H. H. and J. I. P. are the recipients of a Research Fellowship for Young Scientist and of Postdoctoral Fellowships for Foreign Researchers of JSPS, respectively. The authors are grateful to Ayako Chiba (Iwate University), Hitomi Ito, Keiko Nagami, Kohei Sasaki, Masumi Miyano, Rui Sato, Tamae Maruoka, Yui Miyashita and Yukari Wakabayashi (Tohoku University) for technical assistance.
|