| Edited by Toru Terachi. Shigeko Utsugi: Corresponding author. E-mail: utsugi@rib.okayama-u.ac.jp |
In higher plants, the phytohormone abscisic acid (ABA) plays important roles in regulating various processes of plant development, including synthesis of seed storage proteins, promotion of seed desiccation tolerance and dormancy, and inhibition of germination and reproductive growth. ABA is also involved in plant stress responses, inducing stomatal closure under drought and osmotic stresses, or mediating tolerance to salt and cold stresses. Since pre-harvest sprouting (PHS) of seeds is one of the most serious problems in cultivation of cereals, wheat in particular, it is important to understand the mechanism of seed dormancy regulated by ABA.
Maize viviparous1 (vp1) mutant seeds germinate precociously due to the reduced sensitivity to ABA in developing embryos (McCarty et al., 1989). Molecular genetic studies revealed that VP1 is important for the completion of seed maturation (McCarty et al., 1989, 1991; Giraudat et al., 1992; Hattori et al., 1995). Vp1s from maize and cereals and their orthologues from other plants including Arabidopsis ABA Insensitive3 (ABI3) are known to encode transcription factors that are highly expressed in developing seeds (McCarty et al., 1991; Giraudat et al., 1992; Hattori et al., 1995). The maize and rice VP1s promote the transcription of many ABA-regulated genes in developing aleurone (McCarty et al., 1991; Hoecker et al., 1995; Shen et al., 2001). Maize VP1 also activates the promoter of maize C1 gene that regulates anthocyanin synthesis, by directly binding to a Sph element on the promoter, and indirectly activates the transcription of the promoters of the genes encoding the late embryogenesis abundant proteins (LEAs) such as Em (McCarty et al., 1991; Hoecker et al., 1995). In addition to VP1, basic domain leucine-zipper (bZIP) transcription factors such as EmBP1 or ABI5 are needed for the activation of the Em promoter (Vasil et al., 1995; Schultz et al., 1998; Nakamura et al., 2001; Carles et al., 2002; Himmelbach et al., 2003). Meanwhile, VP1 is also known to down-regulate the α-amylase expression in the aleurone cells of maize and barley (Shen et al., 2001; Hoecker et al., 1999). VP1 is thus regarded as a bifunctional transcription factor, which acts an activator as well as a repressor during the development of seeds (Hoecker et al., 1999; Zentella et al., 2002).
ABI3/VP1 orthologues have been identified in Arabidopsis, maize, rice, oat, bean and carrot and found to contain the functional domains, B1, B2 and B3, which bind to DNA and activate the target promoter (McCarty et al., 1991; Giraudat et al., 1992; Hattori et al., 1994; Bobb et al., 1995; Jones et al., 1997; Shiota et al., 1998). In Arabidopsis, the B1 domain is involved in the physical interaction of ABI3 with bZIP proteins such as ABI5, while the B2 domain enhances the binding of bZIP factors to DNA and also shows a weak, nonspecific binding to DNA. The B3 domain directly binds to DNA and takes part in the ABA-independent activation of the maize C1 gene promoter (Nakamura et al., 2001; Carson et al., 1997; Suzuki et al., 1997). Meanwhile, it has been reported that the B2 and B3 domains are necessary for the ABA-dependent regulation of napA in Brassica napus (Ezcurra et al., 2000).
VP1 regulates not only the proper completion of maturation but also the transition from dormancy to germination in response to ABA, as demonstrated by analyses of a maize vp1 mutant (Robichaud et al., 1980; Neill et al., 1986). Nakamura and Toyama (2001) reported that the expression level of TaVp1 in mature seed embryos of a dormant wheat cultivar Minamino-komugi (Minamino) was higher than that of a non-dormant cultivar Tozan-18. They estimated that the level of the TaVp1 mRNA is positively correlated to seed dormancy in these cultivars. By analyzing the genomic clones of TaVp1 homologues in a hexaploid wheat cultivar Soleil, McKibbin et al. (2002) demonstrated that these homologues could be classified into three classes based on their sequences, which represent single-copy loci in the A, B and D genome designated as TaVp-A1, TaVp-B1 and TaVp-D1, respectively. These homoeologues closely resemble each other, except that each of them has a unique sequence in the 3’UTR region and that TaVp-B1, the homoeologue in the B genome, has a 12-bp deletion in exon I. McKibbin et al. (2002) also reported that missplicing of the pre-mRNA of TaVp1 occurred around the B3 domain, resulting in the production of truncated mRNAs and the generation of stop codons in the ORF. Such missplicing was also detected in the majority of TaVp1 transcripts from diploid and tetraploid ancestral wheats, suggesting that modern wheat Soleil have inherited the structure of TaVp1 from its ancestral species.
In the present study, we first confirmed the types and the frequency of the missplicing of TaVp1 in embryos of a highly dormant hexaploid wheat cultivar Minamino. Then, we analyzed the pattern and the level of TaVp1 expression in the embryos and the function of TaVp1 with respect to seed dormancy in wheat. TaVp1 was preferentially expressed in the seeds, and its mRNA level increased during seed development and maturation. In particular, correctly spliced TaVp-B1 transcripts existed in a substantially high proportion among TaVp1 transcripts in Minamino seeds at late developmental stages and in mature seeds, suggesting that TaVp-B1 is an important gene in this dormant cultivar. Furthermore, an efficient transient gene expression assay using aleurone tissues of diploid wheat revealed that TaVP-B1 activated an ABA-regulated Em promoter and suppressed a GA-regulated α-amylase promoter. These results suggest that TaVp-B1 encodes a transcription factor possessing dual function, i.e. the activation of Em and the repression of α-amylase, for regulating seed maturation and dormancy.
Minamino is a highly dormant cultivar of hexaploid wheat (Triticum aestivum L.) (Nakamura and Toyama, 2001). In the experiment for analyzing TaVp1 expression patterns in immature and maturing embryos, Minamino plants were grown at 15°C in a greenhouse, and their seeds were harvested at different developmental stages ranging from 0 to 60 days after pollination (DAP). Under this condition, the seeds were fully matured and desiccated by DAP 60. The harvested seeds were stored at –20°C until use. For other experiments, seeds of hexaploid wheat cultivars (Minamino, Sanin-1 and Tozan-18) and an early flowering mutant line, KT3-5, of T. monococcum L. cultivar Himalaya were harvested from plants grown in fields of Kurashiki, Japan. Transient gene expression experiments were conducted using the aleurone tissues isolated from mature seeds of KT3-5 (Utsugi et al., 2006).
As materials for RNA extraction, embryos were isolated from mature or immature wheat seeds, and roots and leaves were excised from seedlings grown on moistened filter paper at 20°C under fluorescent light for 7 days. Total RNAs for RT-PCR were extracted from these organs using Trizol reagents (Invitrogen) according to the manufacturer’s manual. First-strand cDNA synthesis was carried out using Superscript First-Strand Synthesis System for RT-PCR (Invitrogen).
To analyze structures of transcripts and identify the splice sites, we amplified the sequences covering exons I-VI using TaVp1-forward 1 (5’-CGCGCAAACCCCGGCTACGAATT-3’), and TaVp1-reverse 1 (5’-CCGCTCGAGCTCAGATGCTCACGGCCATC-3’). The PCR program consisted of 1 cycle of 94°C for 5 min and 35 cycles of 94°C for 30 sec, 60°C for 30 sec and 72°C for 1 min. These TaVp1 primer pairs were designed based on common sequences among the three TaVp1 homoeologues in A, B and D genomes, TaVp-A1, TaVp-B1 and TaVp-D1. The amplified cDNAs were cloned into pGEM-T easy vector (Promega) and sequenced by the DyeDeoxy terminator cycle sequencing method using an ABI PRISM 3100-Avant Genetic Analyzer (PE Applied Biosystem). Nucleotide sequences of the cDNAs were analyzed by GENETYX-MAC (Genetyx).
To detect total TaVp1 mRNA accumulation, we designed the following primers, TaVp1-forward 2 (5’-CAAGTCAGGCAAATATCTGATACGCG-3’) and TaVp1-reverse 2 (5’-CATGCATTCTTCAGCGTCACATCTGA-3’), to amplify the region that extends from exon V to exon VI including 3’UTR (semi-quantitative RT-PCR). For preparing the internal control, the Actin1 sequence was amplified using Act1-forward (5’-ATGGCTGACGGTGAGGACATCC-3’) and Act1-reverse (5’-ACGGCCTGAATTGCGACGTAC-3’). Amplified products were electrophoresed in 2% (wt/vol) agarose gels and stained with ethidium bromide. The products were quantified by using Dorphin-Chemi Image System (Wealtec) and analyzed by the program ImageJ (http://rsb.info.nih.gov). The values obtained for TaVp1 were normalized by the control values for Actin1.
For quantifying the TaVp1 transcripts in developing and mature seed embryos, roots and leaves of seedlings, quantitative RT-PCR (QRT-PCR) was performed using the following primer pairs designed using Primer Analysis Software version 6 (MBI): TaVp1Q-forward (5’-ATTGAGGACATCGGCACATC-3’) and TaVp1Q-reverse (5’-CAGCTCATTGGACCGAACA-3’); ActinQ-forward (5’-CTATGTTCCCGGGTATTGCT-3’) and ActinQ-reverse (5’-AAGGGAGGCAAGAATCGAC-3’). QRT-PCR was carried out using SYBR Premix Ex Taq Kit (Takara) and 7500 Real Time PCR System (PE Applied Biosystems) according to the manufacturer’s manuals. The signal amplified with the Actin primers was used as an internal control. The obtained data were analyzed by RQ study software (PE Applied Biosystems).
To amplify the unique sequences of TaVp-A1, TaVp-B1 and TaVp-D1, we performed RT-PCR by using the primer pairs as described by McKibbins et al. (2002). The PCR programs for TaVp-B1 and TaVp-D1 consisted of 1 cycle of 94°C for 1 min; 5 cycles of 94°C for 45 sec, 70°C for 1 min and 72°C for 1 min; and 30 cycles of 94°C for 45 sec, 65°C for 1 min and 72°C for 1 min. The PCR program for TaVp-A1 was the same as those for TaVp-B1 and TaVp-D1, except that the final step was carried out for 45 cycles instead of 30 cycles.
The Act:TaVp-B1 and Act:HBP1a-1 effector constructs used in the transient assays were synthesized by cloning the rice Actin1 promoter (McElroy et al., 1990) and the fragments containing the TaVp1 (Nakamura et al., 2001) and HBP1a-1 (= EmBP1) (Mikami et al., 1994) coding regions into pUC19 vector, respectively. The rice Actin1 promoter and 5’ untranslated leader sequence (including intron I) of pDM302 (Cao et al., 1992) were cut out as a HindIII fragment and cloned into the HindIII site of the pTH-2 (Niwa et al., 1999). The PCR fragment containing the TaVp1 or HBP1a-1 coding region was cloned into the pBluescript SK+ (Stratagene) T-vector. The entire insert was cut out as a Sal I-Not I fragment and cloned into the Sal I-Not I site of the pTH-2-fused Actin1 promoter. The Act:GAmyb was described in Utsugi et al. (2006).
Aleurone tissues of wheat (KT3-5) mature seeds were prepared for particle bombardment according to the procedure described by Utsugi et al (2006). Twenty pieces of aleurone tissues were placed on each plate and bombarded by a particle gun using 1.6 μm gold particles coated with plasmid DNAs purified by Quiagan tip (Quiagan) as described in the manual of a particle delivery system (Bio-Rad biolistic PDS-1000/ He particle delivery system, Bio-Rad). In an experiment for examining the effects of TaVP1 on the Em promoter, 0.5 μg of an effector construct, either Act:TaVp-B1 or Act:HBP1, and 2 μg of a reporter construct, Em:GUS (pBM113Kp) (Marcotte et al., 1988), were precipitated individually or as a mixture onto 0.75 mg of gold particles. In another experiment for examining the effects of TaVP1 on the α-amylase promoter, 0.1, 1 or 2 μg of an effector construct Act:TaVp-B1 and 2 μg of a reporter construct chosen from Am(–877)IGN or Am(–174)IGN (Jacobsen and Close, 1991) were precipitated on the gold particles as above. Ubi1-LUC was added to each sample in a ratio of 1:1 to the reporter plasmid as an internal control for the normalization of β-glucuronidase (GUS) expression. After bombarding, aleurone tissues were incubated on filter paper soaked with water (control), 10 μM ABA or 1 μM GA3 containing 10 mM CaCl2, 50 U/ml nystatin and 150 μg/ml cefotaxime at 24°C for 48 hours. Preparation of extracts and GUS assays were conducted according to the procedure described by Lanahan et al. (1992). For the quantitative assay using 4-methyl-umbelliferyl β-D-glucuronide (MUG, Sigma), the tissue extract for each treatment was prepared from ten pieces by the method of Utsugi et al. (2006). A 100 μl aliquot of each extract from the aleurone tissues was added to 400 μl lysis buffer containing 1 mM MUG and incubated at 37°C. The reaction was terminated by the addition of 0.2 M Na2CO3 400 μl, and the fluorescence was measured with excitation at 365 nm and emission at 455 nm using a spectrofluorophotometer (RF-5300PC, Shimadzu). The expression of GUS was standardized by LUC expression. Each experiment was replicated at least eight times.
The germination and the effects of ABA were examined by a germination test using seeds of Minamino and non-dormant cultivars Sanin-1 and Tozan-18. The seeds were cut in halves with a cutter blade to break the dormancy (Kawakami et al., 1997). The half-grains having embryos were incubated on filter paper with water or 10 μM ABA solution at 20°C for 14 days. As shown in Fig. 1, all seeds of Sanin-1 and Tozan-18 germinated after imbibition of 2 to 3 days both in water and in ABA. All seeds of Minamino also germinated in water, although they germinated 1 to 2 days later than those of the non-dormant cultivars. In ABA, however, only 53% of Minamino seeds germinated within 2 to 4 days after the seeds of the non-dormant cultivars had germinated, and the rest (47%) did not germinate. Our result suggests that Minamino embryos are much more dormant and sensitive to the germination inhibitory action of ABA than those of the non-dormant cultivars.
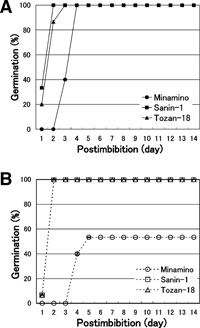 View Details | Fig. 1 Comparison of germination frequencies of mature seeds between a dormant cultivar Minamino and non-dormant cultivars Sanin-1 and Tozan-18. Seeds at DAP 50 were cut, and the half-grains having embryos were incubated on filter paper with water (A) or 10 μM ABA solution (B) at 20°C for 14 days in the dark. The experiment was repeated three times by using 10 seeds of each cultivar, and the percentage of germination was determined by dividing the number of germinated seeds by the total number of seeds. The percentages of germination are indicated by symbols ●, ■ and ▲ for Minamino, Sanin-1 and Tozan-1 in water, respectively, and ○, □ and △ for these cultivars in ABA, respectively. Bars indicate the standard errors. |
McKibbin et al. (2002) has reported that the majority of pre-mRNAs transcribed from the TaVp1 homoeologues were spliced incorrectly. To examine whether the TaVp1 pre-mRNAs accumulated in Minamino were spliced correctly or incorrectly, we conducted RT-PCR using mRNA samples prepared from embryos at DAP 20, 40 and 60 with the primer pair (TaVp1-forward 1 and -reverse 1) that amplifies the region including exons I through VI and all introns. As shown in Fig. 2, we found a band potentially containing misspliced transcripts at DAP 20. This corresponds to Type III transcripts as described later (Fig. 3B).
 View Details | Fig. 2 TaVp1 transcripts in embryos of Minamino seeds at DAP 20, 40 and 60. 0.1 μg of total RNA from each set of the tissues was reverse-transcribed and amplified by PCR. A cDNA from TaVp-B1 was also amplified as a positive control for PCR (C). The black and gray arrows indicate correctly and incorrectly spliced transcripts, respectively. |
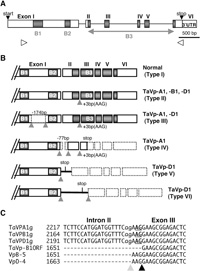 View Details | Fig. 3 Structures of TaVp1 gene and its transcripts in Minamino. (A) Schematic representation of TaVp1 gene structure. ORFs are indicated by boxes and B1, B2 and B3 domains by shaded squares. White arrowheads indicate the primer pair (TaVp1-forward 1 and -reverse 1) used to amplify a region including exons I through VI and all introns. (B) Structures of correctly spliced (Type I) and misspliced (Type II–VI) TaVp1 transcripts. Arrows indicate the missplice sites. Missplice sites at three bases (AAG) upstream of the standard site, deleted regions (boxes surrounded by dotted lines) and their sizes (bp) are indicated. (C) The sequence alignment of a misspliced region of Type II transcripts. TaVPA1g, TaVPB1g and TaVPD1g are genomic sequences of the TaVp1 genes in the A, B and D genomes, respectively, TaVp-B1ORF is the standard cDNA sequence of TaVp-B1 (GeneBank accession no. AB047554), and VpB-5 and VpD-4 show Type II transcripts derived from B and D genome in Minamino, respectively. VpB-5 and VpD-4 each possesses additional AAG in exon III. Underlines indicate the recognition sites for correct splicing of intron II at its 3’-end in TaVp1 genomic sequence. For the 3-bp inserted transcripts, the putative recognition site for splicing is indicated by small letters. The black and gray arrowheads indicate correctly and incorrectly spliced transcripts, respectively. |
We cloned these RT-PCR products amplified from the mRNA samples of DAP 20 to 60 and sequenced the cDNA clones to assign them to the individual homoeologues and to examine the splicing patterns. Table 1 shows the proportions of the clones assigned to the individual TaVp1 homoeologues. In the embryos from DAP 20 to 60, the clones corresponding to TaVp-B1 and TaVp-D1 totally accounted for 80 to 90%. By contrast, the proportion of TaVp-A1 clones was distinctly lower and decreased to less than 20%. However, the PCR amplification efficiencies of the mRNAs and the cloning efficiencies of the PCR products may differ among the homoeologues, and thus the proportions of the cDNA clones may not reflect the original proportions of the homoeologous mRNAs directly. For this reason, we could not critically estimate the proportions of the homoeologous transcripts. Therefore, the result only indicates that the TaVp-A1 cDNAs derived from the mRNAs of the developing seeds embryos (DAP 20) were more easier to clone than those from maturing seed embryos (DAP 40 to 60), and that TaVp-B1 cDNA clones were most abundantly obtainable from mRNAs of the maturing seeds (DAP 40), while TaVp-D1 clones were constantly obtainable as the seeds developed (DAP 20 to 60).
 View Details | Table 1 Proportion of transcripts from each homoeologue to total TaVp1 transcripts in developing and matured embryos in a dormant wheat variety Minamino |
By comparing the sequences of clones derived from TaVp-A1, TaVp-B1 and TaVp-D1 transcripts, six types of transcripts were distinguished among the cloned cDNAs (Fig. 3). Type I was produced by correct splicing, and Type II was produced by splicing at a site 3-bp (AAG) upstream of the standard site between intron II and exon III (Fig. 3B). Almost all of the TaVp-D1 transcripts (96%) and some of the TaVp-A1 and TaVp-B1 transcripts (29 and 67%, respectively) were assigned to Type II (Table 1). The protein derived from a Type II transcript would possess one amino acid residue (lysine) added to the B3 domain of the protein from Type I. In this case, the spliceosome may recognize the AG sequence that is located 3-bp upstream of the original recognition site as the splice site (Fig. 3C). Types III and IV were new types found in Minamino. Type III transcripts were generated by splicing both ends of a 174-bp (58 amino acids) region within exon I (Fig. 3B). The protein products of the Type III transcripts may not function as transcription factors, because they lacked most of the region (310–367 amino acids) between the B1 and the B2 domains, which perhaps would result in extensive alterations in their protein structure (Hoecker et al., 1995). Type III transcripts, which were detected as a single band that moved slightly faster than the main band in the gel (Fig. 2), accounted for 57% and 22% of the transcripts derived from TaVp-A1 and TaVp-D1 at DAP 20, respectively, but were not detected among the TaVp-B1 transcripts. As shown in Fig. 4, both 5’ and 3’ missplice sites of Type III were in the sequences constituting a short inverted repeat, which may have affected the configuration of the pre-mRNA to induce missplicing. One nucleotide difference was found in the 5’ sequence of the inverted repeat of TaVp-B1 (Fig. 4), but it is not clear whether this had caused the difference in missplicing frequencies between TaVp-B1 and other homeologues. Type IV, V and VI transcripts were the products of incorrect splicing around intron I and completely lacked the B3 domain. Type IV transcripts were derived from only TaVp-A1 and lacked a total of 77 bp (52-bp in exon I and 25-bp in exon II) in a region in the ORF divided by intron I. Missplice sites were found in the sequences constituting another inverted repeat (Fig. 4), and a frameshift in the reading frame generated a premature stop codon in the middle of exon III (Fig. 3B). Type V transcripts included the latter half (139-bp) of intron I due to the emergence of a misspliced 3’ end in intron I, while Type VI transcripts included the full sequence of intron I due to missplicing. Type V and VI transcripts carried respective stop codons in the sequence of intron I. Such missplicing events appear to occur easily in TaVp-D1. The stop codons in Type IV, V and VI transcripts would terminate translation and produce truncated proteins. Most of the TaVp-A1 transcripts at DAP 20 were non-functional Type III and IV transcripts. However, it is unclear why no misspliced TaVp-A1 transcripts were detected at DAP 40 and 60 (Table 1). About 10 to 22% of the TaVp-D1 transcripts were also misspliced in the embryos at DAP 20 to 60 (containing Type III, V and VI transcripts) (Table 1). We could not detect any misspliced TaVp-B1 transcripts except Type II at any stage (total of 58 clones of TaVp-B1). No remarkable differences in the genomic sequences surrounding these missplice sites were detected between TaVp1 homoeologues, while significant differences were detected in the sequences of intron I. This suggests that the sequences of intron I may be responsible for the various missplicing events in each homeologue.
 View Details | Fig. 4 Alignment of the partial TaVp1 genomic DNA and cDNA sequences. TaVPA1g, TaVPB1g and TaVPD1g are genomic sequences of the TaVp1 genes cloned from Minamino. VpB-ORF shows the cDNA sequence of TaVp-B1 (GeneBank accession no. AB047554), and VpA-2 (III) and VpD-11 (III) show Type III transcripts derived from A and D genomes, respectively. VpA-9 (IV) and VpD-18 (VI) show Type IV transcripts derived from A genome and Type VI transcripts derived from D genome, respectively. Small letters are sequences of the intron, and broken lines indicate the exon-intron boundaries. Arrowheads indicate the missplice sites in each transcript. Arrows indicate inverted repeats (IR) around splice regions, and the complemented nucleotides are marked by dots. One B genome-specific nucleotide T is shown by a shaded square in the 5’-IR of Type III transcript. Single and double underlines indicate a reverse repeat and a B genome-specific tandem repeat, respectively. The boxed TAA is a premature stop codon generated by missplicing. |
As described above, the majority of the TaVp1 pre-mRNAs in Minamino embryos were correctly spliced transcripts (Fig. 2 and Table 1). To confirm the tissue-specificity of the TaVp1 expression, RT-PCR was performed using RNA samples prepared from embryos of mature seeds, roots and leaves of 7-day-old seedlings of Minamino. As shown in Fig. 5A, TaVp1 was highly expressed in the embryos of mature seeds, while it was only slightly expressed in roots and leaves. To examine the temporal expression pattern of TaVp1 during seed development, RT-PCR was carried out by using RNA samples prepared from ovaries collected just after pollination (DAP 0) and embryos at DAP 20, 40, 50 and 60. TaVp1 was not expressed at DAP 0 but was distinctly expressed, at the latest, after DAP 20 (Fig. 5A). The results of QRT-PCR showed that the level of the TaVp1 transcripts reached a maximum at DAP 50 and decreased during ripening to maturity (DAP 60) (Fig. 5B).
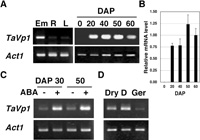 View Details | Fig. 5 Seed-specific expression of TaVp1 in Minamino. (A) RT-PCR products from TaVp1 mRNAs derived from embryos of mature seeds (Em), roots (R) and leaves (L) of 7-day-old seedlings (left), and RT-PCR products from TaVp1 mRNAs derived from ovaries (DAP 0) and embryos at DAP 20, 40, 50, and 60 (right) by semi-quantitative RT-PCR. One μg of total RNA from each set of the tissues was reverse-transcribed. Single-strand cDNA was used as the template for PCR using a primer pair for amplifying the TaVp1 sequences. Wheat Actin1 transcript (Act1) was amplified as a control to quantify the constitutive expression level. (B) TaVp1 mRNA levels in embryos at various seed developmental stages determined by QRT-PCR. Actin1 mRNA was used as an internal control. The relative mRNA level is the ratio of the measured amount of mRNA in each sample to that in the DAP 60 sample. (C) TaVp1 mRNA levels in embryos exposed to ABA by semi-quantitative RT-PCR. Sets of half-grains having embryos prepared from seeds at DAP 30 and DAP 50 were incubated on filter paper with water (–) or 10 μM ABA solution (+) at 20°C for 48 hours (left). (D) RT-PCR products from TaVp1 mRNAs in embryos of dormant and dormancy-broken seeds. Intact seed grains (DAP 50) were incubated on filter paper soaked with water at 20°C for 14 days (dormant seeds, D) or at 4°C for 7 days and subsequently at 20°C for 1 day (germinating seeds, Ger) (right). The embryos of dry seeds were used as controls (Dry). Total RNA was isolated from each set of embryos to synthesize the cDNA used for semi-quantitative RT-PCR. |
Minamino seeds have been shown to be much more dormant and sensitive to ABA than those of the non-dormant cultivars (Fig. 1). To confirm the correlation between the TaVp1 mRNA level in embryos and the ABA sensitivity, half-grains prepared from Minamino seeds at DAP 30 and 50 were incubated with water or ABA solution, and RT-PCR was carried out using the RNAs extracted from the embryos. The results showed that a larger amount of TaVp1 mRNA was accumulated in ABA-treated embryos than in water-treated controls at both DAP 30 and 50 (Fig. 5C).
As another treatment to break seed dormancy, whole seeds were incubated with water at 4°C for several days, and the levels of TaVp1 mRNAs in embryos of dormant and dormancy-broken seeds were compared. Mature seed grains were incubated on filter paper soaked with water at 20°C for 14 days (dormant seeds) or at 4°C for one week and subsequently at 20°C for 1 day (dormancy-broken seeds), and the total RNA was isolated from each set of the embryos. As a result of RT-PCR, much higher levels of TaVp1 mRNA were found in the embryos of the dormant seeds than in those of the dormancy-broken seeds (Fig. 5D). This indicates that the TaVp1 mRNA level decreases as the embryos shift to the germination stage. These results suggest that TaVp1 encodes a factor for maintaining dormancy and repressing germination of wheat seeds.
The temporal expression pattern of each TaVp1 homoeologue (TaVp-A1, TaVp-B1 or TaVp-D1) during seed development was examined by RT-PCR. The primer pair designed based on the specific sequence of each homoeologue was used to amplify the corresponding transcript by RT-PCR (McKibbin et al., 2002). TaVp-A1 products could not be detected when PCR was carried out at the condition described by McKibbin et al. (2002), but after additional 15 cycles, they could be clearly detected in embryos at DAP 20 and, at very low levels, at DAP 40 to 60 (Fig. 6). TaVp-B1 products were most abundant at DAP 40 and gradually decreased as the embryos matured. The amount of TaVp-D1 transcripts was first comparatively low but increased as the embryos developed, reaching a maximum in mature seeds.
 View Details | Fig. 6 Semi-quantitative RT-PCR analysis of TaVp1 homoeologues in Minamino. Total RNAs were isolated from ovaries (DAP 0) and embryos at DAP 20, 40, 50, and 60. RT-PCR was carried out by using the specific primer pair for amplifying each TaVp1 homoeologue, TaVp-A1, TaVp-B1 or TaVp-D1. Actin1 transcript (Act1) was amplified as a control to quantify the constitutive expression level. The PCR conditions of the homoeologues were described in Materials and Methods. |
TaVp1 transcripts having the inserted AAG were probably functional, because such an additional AAG is commonly present in the ABI3/Vp1 mRNA of most plants except Avena fatua (Jones et al., 1997). As mentioned above, among the TaVp1 homoeologues, TaVp-B1 and TaVp-D1 were highly expressed in embryos of mature seeds, implying that they are important TaVp1s that function to maintain the dormancy and suppress the germination in hexaploid wheat. Especially, TaVp-B1 was predominantly expressed in embryos throughout the late developmental and maturing seed stages. We conducted an efficient transient assay using aleurone tissues from seeds of a diploid wheat line, KT3-5, to examine whether the TaVp-B1 product could transactivate a construct Em:GUS containing a GUS gene driven by the promoter of Em, one of the ABA-responding genes in wheat (Fig. 7A). As an effector construct, we used Act:TaVp-B1 expressing the TaVP-B1 (Type I transcript) protein (Fig. 7A). In order to compare the effect of TaVP-B1 on the activation of Em promoter with that of a bZIP transcription factor, we used the construct Act:HBP1a-1 expressing HBP1a-1 (EmBP1) in wheat, which is known to bind to Em promoter as an effector (Fig. 7A). The aleurone tissues were co-bombarded with the Em:GUS reporter construct or a mixture of this reporter construct and one or two effecter constructs, Act:TaVp-B1 and/or Act:HBP1a-1. The bombarded tissues were incubated on filter paper soaked with water or 10 μM ABA solution. The GUS activity was standardized by LUC expression.
 View Details | Fig. 7 Activation of Em expression by TaVP1 in a transient gene expression system. (A) Structures of the effector and reporter constructs. Em:GUS (pBM113Kp) is a reporter construct having a wheat Em promoter fused to GUS and NOS reporter cassette. GUS: the β-glucuronidase coding sequence, NOS: the nopaline synthase terminator sequence. Act:TaVp-B1, Act:HBP1a-1 and Act:GAmyb are effector constructs with TaVp-B1, HBP1a-1 and GAmyb coding sequences, respectively, inserted between the rice Actin1 promoter and the NOS terminator. (B) Effects of effectors Act:TaVp-B1 and Act:HBP1a-1 on activation of Em promoter. Aleurone tissues were bombarded with Em:GUS alone or co-bombarded with a mixture containing Em:GUS and either or both Act:TaVp-B1 and Act:HBP1a-1. The experiment was repeated at least eight times. Mean values of the GUS activities in the tissues incubated on filter paper soaked with ABA solution and in water are presented by black (ABA) and open (water) columns, respectively. The error bars indicate standard errors of the means. + and – below the graph indicate the presence and absence of each construct, respectively. Act:GAmyb was used as a positive control for the assay. |
As shown in Fig. 7B, ABA increased the GUS expression in Em:GUS-transformed tissues (5-fold increase). In the absence of ABA, the GUS activity was also enhanced by the TaVP-B1 effector to reach a level close to that achieved in the presence of ABA. This result indicates that the TaVP-B1 functions as an activator for Em expression. In tissues co-transformed with Em:GUS and Act:HBP1a-1, high GUS activities were found, both in the absence and presence of ABA (6-fold of that of Em:GUS-transformed tissues treated in water), which were even higher than that enhanced by the TaVP-B1 effector. In addition, in tissues co-transformed with Em:GUS and both Act:TaVp-B1 and Act:HBP1a-1 effectors, the GUS activity was almost equivalent to those achieved through co-transformation with Em:GUS and Act:HBP1a-1. We also examined whether GA-inducible Myb transcription factor (GAMyb) affects Em expression (Fig. 7B). The GUS activity in tissues co-transformed with Em:GUS and Act:GAmyb (Fig. 7A) increased 8-fold by the addition of ABA. This pattern was similar to that observed in the tissues transformed with Em:GUS alone, which suggests that GAMyb is not involved in the regulation of Em expression.
We also tested the effect of TaVP-B1 on the expression of α-amylase encoding a key enzyme involved in GA-regulated seed germination by a transient assay. The reporter construct Am(–877)IGN, containing a GUS gene driven by an –877 region of the α-amylase promoter from the start codon, and the Act:TaVp-B1 effector construct were transferred into aleurone tissues (Fig. 8A). In tissues transformed with the reporter construct alone GA treatment markedly increased the GUS activity, but their co-transformation with 1, 1/2 or 1/20 volume of Act:TaVp-B1 inhibited the activity (Fig. 8B).
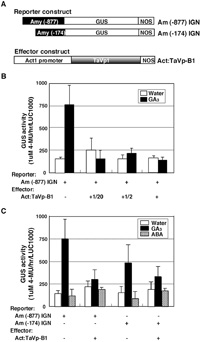 View Details | Fig. 8 Repression of α-amylase expression by TaVP-B1 in a transient gene expression system. (A) Structures of reporter and effector constructs. Am (–877) IGN and Am (–174) IGN are reporter constructs having –877 and –174 regions of a barley high-pI α-amylase promoter, respectively, fused to a GUS and NOS reporter cassette. The effector construct Act:TaVp-B1 is as explained in Fig. 7. (B) Effect of the amount of Act:TaVp-B1 introduced by bombardment on α-amylase promoter activation. Reporter and effector constructs used in co-bombardment experiments are indicated below the graph; + and – indicate the presence and absence of each construct, respectively. –, +1/20, +1/2 and + indicate 0, 1/20, 1/2 and 1 volume of the effector construct per 1 volume of the reporter construct used for bombardment. GUS activities of aleurone tissues incubated on filter paper soaked with GA3 solution and in water are shown by black (GA3) and open (water) columns, respectively. (C) Comparison of α-amylase promoter activities stimulated by TaVP-B1 between two reporter constructs having different promoter lengths. Half a volume each of effector plasmid DNA per one volume of reporter plasmid DNA was used for each experiment. The experiment was repeated at least eight times. Mean values of GUS activity of aleurone tissues incubated in water, GA3 and ABA are shown by open, black and shaded columns, respectively. The error bars indicate standard errors of the means. |
We further analyzed the effect of TaVP-B1 on the α-amylase promoter using two reporter constructs, Act:Am(–877)IGN and Act:Am(–174)IGN (Fig. 8A). In this experiment, wheat aleurone tissues were bombarded with either of these reporter constructs with or without the Act:TaVp-B1 effector construct and subsequently treated with water (control), 10 μM GA3 or 10 μM ABA solution (Fig. 8C). The α-amylase promoter of Am(–174) IGN was long enough to harbor elements for the GA-dependent expression such as GA response element (GARE) and TATA-box (Jacobsen et al., 1991). We compared the effects of TaVP-B1 on the expressions of Am(–174)IGN and Am(–877)IGN. The GUS expression pattern in tissues with Am(–174)IGN was similar to that in tissues with Am(–877)IGN. In tissues bombarded with only Am(–877)IGN or Am(–174)IGN, the GUS activity was markedly increased by GA treatment but unaffected by ABA treatment (Fig. 8C). Such high GUS activity induced by GA, however, was lowered when Act:TaVp-B1 was co-bombarded into the tissues, suggesting that TaVP-B1 suppressed the GUS activity by interfering with the element GARE that exists –174 region of α-amylase promoter. TaVP-B1 is assumed to suppress the expression of α-amylase indirectly rather than directly, by suppressing the expression of GAmyb, which is induced by GA and whose product activates the α-amylase promoter by interacting with GARE or a GARE complex (Gubler et al., 1995).
VP1 has been reported to be a transcriptional activator of many ABA-regulated genes and also a repressor of α-amylase in the cereal aleurone cell to control seed maturation and germination (McCarty et al., 1991; Hoecker et al., 1995; Shen et al., 2001). In hexaploid wheat, it was reported that TaVp1 mRNA was accumulated much more in mature seed embryos of a dormant cultivar than in those of a non-dormant cultivar, suggesting a positive correlation among the levels of TaVp1 transcript, seed dormancy and ABA sensitivity in mature embryos (Nakamura and Toyama, 2001). On the other hand, McKibbin et al. (2002) demonstrated that the majority of pre-mRNAs transcribed from the TaVp1 homoeologues were spliced incorrectly to generate a large proportion of non-functional proteins that might have contributed to the increased PHS in European wheat cultivar Soleil. We were interested in the frequency of alternative splicing in a highly dormant Japanese wheat cultivar Minamino and the relation between the expression and the function of the TaVp1.
Although some misspliced transcripts were found at DAP 20, many transcripts were correctly spliced in maturing embryos of Minamino. At DAP 40 to 60, the majority of TaVp1 mRNAs included correctly spliced functional transcripts and presumably functional transcripts with an inserted AAG in exon III. These results appear to be inconsistent with those reported by McKibbin et al. (2002), in which the majority of TaVp1 transcripts were incorrectly spliced in wheat lines increased PHS. However, these data cannot be directly compared, due to the differences in genetic backgrounds between Minamino and Soleil and in stages of the seeds used for analyzing TaVp1 transcripts: McKibbin et al. used mature imbibed seeds, while we used developing and maturing seeds. McKibbin et al. (2002) also demonstrated that ripening ears of transgenic wheat plants carrying the Avena fatua Vp1 transgene were less susceptible to PHS. From these results, they suggested that missplicing of wheat Vp1 transcripts contributes to susceptibility to PHS. Minamino is, however, one of the most highly dormant wheat cultivars showing a strong resistance to PHS under high humidity typical to the early summer climate in Japan. The ability of Minamino to correctly splice the majority of TaVp1 pre-mRNAs appears to be a specific character that has been established through the process of breeding.
Although the majority of the TaVp1 transcripts were correctly spliced, some misspliced transcripts were also found in developing seed embryos of Minamino. The missplicing events of TaVp1 transcripts were classified into five types based on the results of more detailed sequencing analyses. Exon III of some transcripts, especially those derived from TaVp-D1, possessed an additional AAG at its 5’ end, as it was reported by McKibbin et al. (2002). Because such an additional AAG is commonly present in the ABI3/Vp1 mRNA of most plants except Avena fatua (Jones et al., 1997), TaVp1 transcripts having the inserted AAG were assumed to be also functional. Other four types of transcripts were found to have been incorrectly spliced in exon I or in the regions around intron I. Two of them were transcripts incorrectly spliced or misspliced in intron I as those reported by McKibbin et al. (2002), and the rest was new types found in Minamino. The missplice sites of these two novel types were detected in inverted repeats, suggesting that the inverted repeats might have been one of the factors causing missplicing events. The four types of transcripts possess stop codons in certain ORFs that would lead to production of truncated proteins or deletions between the B1 and B2 domains that might cause structural variations. These missplicing events that would break their functions as transcription factors had apparently occurred in TaVp-A1 and TaVp-D1 but not in TaVp-B1.
The analyses of the TaVp1 expression pattern showed that TaVp1 mRNAs were highly accumulated in the embryos of maturing seeds, as observed for those of ABI3/Vp1 in other plants. They were also accumulated in the embryos of dry seeds and non-germinable imbibed seeds but markedly decreased in germinating embryos. Minamino embryos were proved to be highly sensitive to the inhibitory effect of ABA on germination and to maintain a high TaVp1 mRNA level in response to ABA. These results suggest that TaVP1 plays important roles on response to ABA and seed dormancy. The three homoeologues, TaVp-A1, TaVp-B1 and TaVp-D1, showed different expression patterns. The level of TaVp-B1 mRNA reached a peak in the late developmental stage, and its pattern closely resembled that of total TaVp1 mRNA. On the other hand, the TaVp-D1 mRNA level was markedly low in early developmental stages but gradually increased as the seeds matured. The molecular analyses of TaVp1 transcripts also showed that the TaVp-B1 transcripts accounted for the most of the total TaVp1 transcripts and were spliced correctly in developing Minamino seeds. Wilkinson et al. (2005) has also reported that 69% of RT-PCR clones from wheat variety Chinese Spring were correctly spliced TaVp-B1 transcripts. A structural comparison of cDNAs among the TaVp1 homoeologues revealed that TaVp-B1 possessed a full-length open reading frame with B1, B2 and B3 domains but had a 12-bp deletion in exon I. Despite of this deletion, TaVP-B1 was confirmed to be functional in wheat aleurone tissues by the transient assay of this study. Yang et al. (2007) also presumed the contribution of TaVp-B1 to PHS tolerance. Based on the analyses using 89 white-grained Chinese wheat lines, they reported that two new allelic variants of TaVp1 derived from B genome, which had an insertion of 193-bp or a deletion of 83-bp in intron III, seemed to be related to PHS tolerance. However, there were no indels in intron III of TaVp-B1 or other homoeologues in Minamino (data not shown), and we could not detect any TaVp1 transcripts misspliced in intron III. Therefore, the resistance to PHS in Minamino should be attributed to some other factors rather than the incorrect splicing of TaVp-B1 in intron III.
Arabidopsis ABI3 protein was demonstrated to interact physically with ABI5, a bZIP protein, through the B1 domain and to enhance the DNA binding activity of ABI5 by the function of the B2 domain (Nakamura et al., 2001). Although the ABI3/VP1 protein does not directly bind to the ABA regulatory element (ABRE), it can activate the expression of certain ABA-regulated genes, such as the Em gene encoding one of the LEA proteins, by forming complexes with bZIP transcription factors, such as EmBP1 or ABI5, and 14-3-3 proteins, and enhancing their DNA-binding activities (Carles et al., 2002). To investigate the functions of TaVP1 using the transient expression system, we employed a TaVp-B1 clone (Type I transcript) as an effector construct, since TaVp-B1 appeared to be most predominant among the TaVp1 homoeologues. In our transient expression experiments using wheat aleurone tissues, TaVP-B1 activated the Em promoter without ABA treatment, suggesting that TaVP-B1 was a transcriptional activator of Em expression. In addition, HBP1a-1 introduced into aleurone tissues could activate the Em promoter to a level almost equivalent to that achieved by introducing both TaVP-B1 and HBP1a-1. Since we have confirmed that HBP1a-1 and some other wheat HBP homologues encoding bZIP proteins are expressed in wheat seeds (data not shown), these bZIP protein(s) should be essential for the binding of TaVP1 and other factors to ABRE on the promoters of ABA-inducible genes in the wheat aleurones. The higher Em promoter activation effect of HBP1a-1 compared to that of TaVP-B1 suggests that HBP1a-1 is epistatic to TaVp-B1. This supports the report of Lopez-Molina et al. (2002) who demonstrated that ABI5 acts downstream of ABI3 in Arabidopsis.
Hoecker et al. (1999) reported that, in maize, α-amylase expression in aleurones is enhanced in developing embryos homozygous for vp1 that blocks ABA signal transduction or for vp5 that blocks ABA synthesis. They also found that α-amylase expression was similarly enhanced in aleurones of seeds homozygous for emb-2008 that blocks embryo development at early transition stage (Hoecker et al., 1999). They proposed that the quiescent embryos of wild type seeds release an inhibitory signal to the aleurone. The accumulated Vp1 transcripts are supposed to be at least a part of the inhibitory signal that suppresses the germination of ripening cereal seeds. Hoecker et al. (1999) and Shen et al. (2001) reported that α-amylase expression in the aleurone cells is down-regulated by VP1 in maize and barley. In the assay of maize aleurone tissues, the α-amylase promoter activity was inherently high (Hoecker et al., 1999). However, compared to maize, the α-amylase promoter activity was detected in much lower level by a transient assay used wheat aleurone tissues under hormone-free condition. Therefore, we introduced the TaVp-B1 construct into aleurone tissues and treated them with GA to stimulate α-amylase expression. This experiment clearly confirmed the suppressive effect of TaVP-B1 on α-amylase expression. Thus, TaVP-B1 potentially controls the α-amylase expression through its interaction with GA-induced transcription factors or regulation of their expression.
VP1 is known to interact with the G-box element of the Em promoter and to regulate the expression of target genes through a bZIP protein (Vasil et al., 1995). The induction of α-amylase expression is controlled through interactions of regulatory proteins with cis-acting elements such as GARE in the α-amylase promoter (Rogers et al., 1994). The high-pI α-amylase promoter in wheat seeds contained two G-box core sequences: one in a –500 upstream region of the start codon and the other in GARE. In our transient expression experiment using two reporter constructs, i.e. Am(–877)IGN possessing these two G-boxes in the –500 region and in GARE of the promoter and Am(–174)IGN possessing only the one in GARE, the length of the promoter regions did not affect the activity of TaVP-B1, suggesting that TaVP-B1 regulates the α-amylase expression through either its direct binding to GARE, or its interaction with other factor(s) bound to GARE or the GARE complex, rather than its binding to the G-box in the –500 region. This result also suggests that TaVP-B1 may directly or indirectly suppress the induction of GAmyb expression by GA or inhibit the functions of other GA-responsive factors to inactivate the α-amylase promoter. It has been reported that GAMyb is a transcription factor that specifically binds to a TAACAAA box in GARE in the α-amylase promoter and activates the gene expression in barley (Gubler and Jacobsen, 1992; Gubler et al., 1995). A Dof protein in rice, OsDOF3, was reported to be a transcription factor that binds to a pyrimidine box on the promoter of a GA-response gene, RAmy1A, physically interacts with rice GAMyb and induces a high expression level of this target gene in aleurones (Washio, 2001, 2003). Although it is still unclear how TaVP-B1 suppresses the α-amylase expression and whether additional factors such as GAMyb and Dof proteins are necessary for this regulation, it is clear that TaVP-B1 down-regulates the gene expression through the –174 region of the α-amylase promoter in wheat aleurone tissues.
Several factors interacting with VP1 have been identified, e.g. EmBP1 (HBP1a-1) and other bZIP proteins that are expressed in seed-specific manner. In addition, the amounts of ABA and GA and their balance in seeds are important factors controlling seed development, maturation and germination. Recently, it was reported that ABA 8’hydroxylase plays important roles in controlling the ABA levels in Arabidopsis and barley (Kushiro et al., 2004; Millar et al., 2006). In addition, the cross-regulation of ABA- and GA-signal transductions would also be an important mechanism controlling the development of seeds. An ABA-induced protein kinase, PKABA1, has been reported to mediate the suppression of α-amylase expression by ABA (Gomez-Cadenas et al., 2001). Based on the findings that PKABA1 down-regulated the GAmyb expression and that ABA acted similarly on slender mutation and the wild type, they suggested that PKABA1 acts upstream of the action of GAMyb but downstream of that of the SLENDER1, which is a putative negative regulator of GA signaling. Zentella et al. (2002) suggested that there are two independent ABA signaling pathways that lead to the suppression of α-amylase: one dependent and the other independent on PKABA1. However, details of the acting positions of VP1 and PKABA1 on the GA signaling pathway and the involvement of VP1 in these PKABA1-dependent and -independent routes are not clear. How the ABA and GA signaling pathways are regulated by VP1 remains to be elucidated.
We would like to thank Dr. J. Jacobsen and Dr. R. S. Quatorano for kindly providing the Am(–877)IGN, the Am(–174)IGN and the pBM113Kp. We are grateful to Dr. Meshi for providing a HBP1a-1 cDNA clone.
|