| Edited by Yoshio Sano. Kazuhiko Noda: Corresponding author. E-mail: knoda@rib.okayama-u.ac.jp |
Phytohormone abscisic acid (ABA) plays an important role in plant responses to biotic and abiotic stresses and in maturation and dormancy development of seeds (Leung and Giraudat, 1998; Finkelstein et al., 2002). Information about the ABA signaling pathway, including ABA receptors, is increasing (Grill and Christmann, 2007). ABA signaling includes transcription factors at the end of the pathway, such as AtABI (abscisic acid-insensitive)3 (the B3 domain-type), AtABI4 (the Apetala2-type), and AtABI5 (the basic leucine zipper (bZIP)-type, Finkelstein et al., 2002). These transcription factors regulate the expression of ABA-responsive genes. In particular, AtABI5 is an ABA-responsive gene and acts in cooperation with AtABI3 as a critical factor in the maturation, dormancy development of seeds, and the dehydration tolerance of young seedlings of Arabidopsis (Finkelstein et al., 2002; Stone et al., 2006).
These transcription factors act as positive regulators in ABA signaling, and their mutants exhibit insensitivity to ABA. Recently, negative regulators have been found. The negative regulators, such as AIP2 E3 ligase for ABI3 (Zhang et al., 2005), RING E3 ligase (Stone et al., 2006) and ABA insensitive five binding protein (AFP) for ABI5 (Lopez-Molina et al., 2003), were involved in the proteolysis of ABI3 and ABI5 proteins. ABA signaling activity is influenced by these positive and negative factors (Lopez-Molina et al., 2003; Stone et al., 2006). Among these negative regulators, Arabidopsis AFP (AtAFP) was reported to function in the developing seeds and young seedlings in which AtABI5 expression was detected (Lopez-Molina et al., 2003).
In wheat, ABA signaling is the main factor in the development of seed dormancy (Kawakami et al., 1997; Walker-Simmons, 1987). According to studies investigating the chromosomal location and the quantitative trait loci (QTL) of the dormancy genes of wheat, many genes that act positively or negatively in the formation of dormancy have been reported and located on the chromosomes of homoeologous groups 2, 3, 4 and 5 (Anderson et al., 1993; Flintham et al. 2002; Noda et al., 2002). Wheat Vp1 genes on the chromosomes of homoeologous group 3, orthologs of AtABI3, have been isolated and might function as a positive regulator for the development of grain dormancy (Nakamura and Toyama 2001; Wilkinson et al. 2005; Yang et al. 2007). TaABF, an ortholog of AtABI5, was also reported to be involved in the maintenance of grain dormancy during imbibition (Johnson et al. 2002). These results indicate that ABA signaling pathway found in Arabidopsis plays an important role in the development of wheat grain dormancy. Negative regulator in ABA signaling is also possible to play an important role in wheat. We isolated three wheat AFP genes (TaAFPs), which were located on the short arms of chromosomes 2A, 2B, and 2D, and named TaAFP-A, -B, and -D, respectively.
Wheat is a hexaploid species with three genomes, A, B and D and in general, contains triplicated homoeologs from each genome. Shitsukawa et al. (2007) showed that the homoeologs of MADS box gene of wheat differentiated during wheat evolution and played differently. We are also interested in the function of three TaAFP homoeologs.
TaAFP was ascertained to consist of one intron and two exons, which included a nuclear localization domain in the middle of the deduced amino acid sequence and an ABI5-binding domain in the C-terminal region. The structure of TaAFP was similar to that of the AtAFP gene. In comparison to AtAFP, TaAFPs were expressed in a greater variety of tissues, such as leaves, roots, and seeds. In these tissues, AtABI5 homologues of wheat (TaABI5) were also expressed. In addition to ABA, salt and dehydration stresses upregulated TaAFP expression.
Triticum aestivum cv. Chinese Spring (CS); nullisomic-tetrasomic (nulli-tetra) lines, in which a pair of homologous chromosomes were substituted with their homoeologous chromosomes; ditelosomic (ditelo) lines, which lack the long or short arm of a specific chromosome; and deletion lines, which lack a part of a specific chromosome arm, were grown under a semitransparent plastic roof in a field (Table 1). Spikes were tagged at pollination, and developing grains were collected at 5-day intervals from pollination to 50 days after pollination (DAP). Grains collected at 5 and 10 DAP, and embryos dissected out from the grains at 15 to 50 DAP, were frozen in liquid nitrogen and stored at –80°C (superfreezer; Sanyo Biomedical, Tokyo, Japan). Young leaves and roots were collected from the seedlings grown under the following conditions. Ten grains were incubated in a cavity (diameter, 2.0 cm; depth, 1.0 cm) with a sieve net at the bottom of a polystyrene foam block floating on aerated water for 7 days. Flag leaves were collected from plants at the flag leaf stage.
 View Details | Table 1 Wheat lines used in the experiments and their chromosomal constitutions of homoeologous group 2 |
Young seedlings at day 7 after germination were incubated in 10–4 M ABA (± cis, trans-abscisic acid; Sigma Chemical Co., St. Louis, MO, USA) at 25°C for 1, 3, 6, and 12 h. In the salt treatment, 200 mM NaCl was used. For dehydration, seedlings were placed in a plastic box (20 × 30 × 10 cm) with 300 g of dry silica gel. Roots were collected after each treatment, frozen in liquid nitrogen, and stored at –80°C.
Genomic DNA was extracted from 2 g of the seedlings grown in the dark at 25°C for 7 days by a previously described method (Murray and Thompson, 1980). Total RNA was extracted from 3 g of whole grain at 5 and 10 DAP, 500 mg of embryos from grains at 15 to 50 DAP, and 1 g of roots and leaves by a previously described method (Himi and Noda, 2004).
cDNA was synthesized according to the protocol of Superscript III (Invitrogen Co., Carlsbad, CA, USA), treated with TthRNaseH (TOYOBO Co., Osaka, Japan), and stored at –20°C. To standardize the amount of cDNA of the samples for semiquantitative RT-PCR, the concentration of ubiquitin cDNA was estimated by the amount of PCR products amplified with ubiquitin primers (Joshi et al., 1991) (Table 2). Expression levels of TaAFPs were estimated by PCR at different cycle numbers with TaAFP-32LP and TaAFP-22RP primers designed in the flanking regions of deletion, which allowed for differentiation of three TaAFP genes (Table 2).
 View Details | Table 2 Names and sequences of the primers designed for amplification of TaAFPs, TaABI5s and ubiquitin |
Genomic DNA (60 μg) was digested with XbaI or BclI restriction enzymes. DNA was separated in a 0.7% agarose gel by electrophoresis at 20 V for 16 h, then at 10 V for 10 h, and at 5 V for 12 h. DNA was transferred to a Hybond N+ membrane (GE Healthcare Bioscience Co., Tokyo, Japan), and incubated in prehybridization buffer (50% [v/v] formamide, 5 × SSC, 0.1% [w/v] N-lauroylsarcosine, 0.02% sodium dodecylsulfate [SDS], 2% blocking reagent [Roche Diagnostics, Tokyo, Japan]) at 42°C for 1 h. The membrane was incubated in the hybridization buffer with the DIG-labeled TaAFP probe at 42°C for 16 h. The TaAFP probe was synthesized with the PCR DIG labeling mix (Roche Diagnostics), and TaAFP-LP and TaAFP-RP primers (Table 2).
Wheat expressed sequence tag (EST) sequence, whsl18m22 (GenBank accession no. BJ290096), and putative rice AFP (OsAFP, BAC84471), which has a sequence similar to that of Arabidopsis AFP (AAF67775), were found in the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov/) using the Basic Local Alignment Search Tool (BLAST). The whsl18m22 sequence was derived from the cDNA of wheat grain collected at 30 DAP. Primers designed from the whsl18m22 sequence are listed in Table 2, and their positions are depicted in Fig. 1. The PCR conditions were as follows: 5 min denaturation at 94°C; then 30 cycles of 30 s at 94°C, 30 s at 58°C, and 1 min at 72°C. We determined the annealing temperature for every primer set after trial PCR using a thermal cycler (PC707; ASTEC, Fukuoka, Japan). PCR products were cloned using the pGEM-T vector system (Promega Co., Tokyo, Japan). The DNA sequences were determined in more than one clones using the ABI 3100 sequencer (Applied Biosystems, Foster City, CA, USA). The sequence data were analyzed using Genetyx version 8.0 software (GENETYX Co., Tokyo, Japan).
 View Details | Fig. 1 Structures of TaAFP genes and relative primer positions used for their isolation by PCR and InvPCR. A. positions of the primers used for TaAFP-A amplification and sizes of the PCR products. B. positions of the primers used for TaAFP-B amplification and sizes of the PCR products. C. position of the primers used for TaAFP-D amplification and sizes of the PCR products. Triangle, primer position; Arrow head, site for restriction enzyme; Dashed line, untranslated region; Box, exon; Solid line: PCR product with size. |
Three primers (TaAFP-6LP, TaAFP-6RP, and TaAFP-Rra) for InvPCR were designed in the whsl18m22 sequence (Table 2, Fig. 1). Genomic DNA of CS was digested with HindIII, SphI or SpeI and self-ligated with a Ligation High DNA ligation kit (TOYOBO Co., Osaka, Japan) (Fig. 1A, 1B and 1C). In the DNA digested with HindIII or SphI, two TaAFP sequences (parts of TaAFP-B and TaAFP-D) were amplified with TaAFP-LP and TaAFP-6RP primers in the first round of PCR and then with TaAFP-6LP and TaAFP-Rra primers in the second round of PCR (Fig. 1B and 1C). For isolation of the 5’ region of TaAFP-A, genomic DNA digested with SpeI was used as a template. The first round of PCR was conducted with TaAFP-LP and TaAFP-Rra primers, and the second round of PCR with TaAFP-6LP and TaAFP-9RP primers (Table 2, Fig. 1A). The transcription factor binding element in the 5’ regions of the TaAFP genes was searched for using the Motif program (http://motif.genome.jp/).
We designed primers (Table 2) based on the wheat EST sequence, whsl18m22, which was similar in deduced amino acid sequence to Arabidopsis AFP (AtAFP) and putative AFP of rice (OsAFP). A PCR product (669 bp) that was amplified in the cDNA from the embryos of CS collected at 30 DAP with TaAFP-LP and TaAFP-RP primers was similar (99.2%) to the whsl18m22 sequence. Three TaAFP sequences, TaAFP-A (3289 bp, AB360911), TaAFP-B (3922 bp, AB360912), and TaAFP-D (3128 bp, AB360913), were obtained by PCR and InvPCR, using genomic DNA of CS as a template and primers designed newly in the sequences obtained in the process of TaAFP isolation (Fig. 1).
A PCR product (2505 bp) amplified in the DNA digested with HindIII by InvPCR included 87 bp of intron sequence, which was identical to a part of the intron sequence of TaAFP-B (Fig. 1B). To confirm that the 2502 bp sequence is a part of TaAFP-B, PCR was conducted with the TaAFP-10LP primer newly designed in the 5’ region and the TaAFP-2RP primer in exon2 (Table 2, Fig. 1B). The amplified product (2078 bp) was a part of TaAFP-B. We obtained 3922 bp of the TaAFP-B sequence from these overlapped sequences. In the DNA digested with SphI, we obtained 1604 bp of the TaAFP-D sequence after the first and second rounds of PCR, with the same primers used for TaAFP-B isolation (Fig. 1C). PCR with TaAFP-10 LP and TaAFP-5RP primers designed in the intron of TaAFP-D showed that the amplified sequence (1604 bp) included the 5’ region of the TaAFP-D. In total, we obtained 3128 bp of the TaAFP-D sequence (AB360913, Fig. 1C). For isolation of the 5’ region of TaAFP-A, genomic DNA was digested with SpeI. The first round of PCR was conducted with TaAFP-LP and TaAFP-Rra primers, and the second round of PCR with TaAFP-6LP and TaAFP-9RP primers (Table 2, Fig. 1A). The PCR product (1424 bp) included 494 bp of the intron sequence, which was identical to a part of the TaAFP-A intron. The PCR product (1786 bp) amplified with TaAFP-14LP primer in the 5’ region and TaAFP-13RP primer in the intron also showed that the 1424-bp sequence included a part of the 5’ region of TaAFP-A. Through PCR amplification, 3289 bp of the TaAFP-A sequence (AB360911) was obtained.
These sequences consisted of two exons, one intron, and 5’ upstream and 3’ downstream regions. The structure of the TaAFPs was the same as those of AtAFP and OsAFP. Deduced amino acid sequences of TaAFP-A, TaAFP-B, and TaAFP-D were also similar to those of the cDNA sequences of barley AFP (HvAFP, AK248211) and OsAFP (Fig. 2). Compared with AtAFP, the nuclear localization domain (119–133 aa of AtAFP) in the middle of the amino acid sequence and the ABI5 binding domain (284–335 aa of AtAFP) in the C-terminal region were well conserved (at more than 68%) in all AFP genes compared.
 View Details | Fig. 2 Alignment of the amino acid sequences deduced from TaAFPs, AtAFP, OsAFP, and HvAFP. The sequences in boxes represent the nuclear localization domain (119–133 aa of AtAFP) and the ABI5 binding domain (284–335 aa of AtAFP) expected from the structure of AtAFP. AtAFP (Arabidopsis thaliana AFP, AAF67775); OsAFP (Oryza sativa AFP, BAC84471); HvAFP (Hordeum vulgare AFP, AK248211); TaAFP-A (Triticum aestivum AFP on chromosome 2A, AB360911), TaAFP-B (Triticum aestivum AFP on chromosome 2B, AB360912), and TaAFP-D (Triticum aestivum AFP on chromosome 2D, AB360913). |
OsAFP was included in the BAC clone, AP005807, on chromosome 7 of rice, which showed synteny with chromosomes of the homoeologous group 2 of wheat (Ahn et al., 1993). We estimated the number of TaAFPs in the normal wheat line CS, nulli2B-tetra2A (no chromosome 2B; 4 doses of chromosome 2A), and nulli2D-tetra2A (no chromosome 2D; 4 doses of chromosome 2A) by Southern blotting (Fig. 3A). Three fragments (19.5 kbp, 17.8 kbp, and 16.4 kbp) were detected in DNA of CS digested with XbaI (Fig. 3A), as well as in the DNA digested with BclI (data not shown). Nulli2B-tetra2A lacked the 17.8-kbp fragment, and nulli2D-tetra2A did not exhibit the 19.5-kbp fragment. The 16.4-kbp fragment was detected in a denser concentration than the other fragments in nulli2B-tetra2A and nulli2D-tetra2A. These results show that wheat has three TaAFP genes, which are located on chromosomes 2A, 2B, or 2D. To determine which chromosome of homoeologous group 2 each of the TaAFPs was located, TaAFP genes were amplified with primers specific to one of TaAFP-A, TaAFP-B, and TaAFP-D in the ditelo lines of homoeologous group 2 (Fig. 3B). The TaAFP-B fragment (ca. 750 bp) was not amplified with TaAFP-4LP and TaAFP-4RP primers (Table 2) specific to TaAFP-B in ditelo 2BL, which lacks the short arm of chromosome 2B. The results indicate that TaAFP-B is located on the short arm of chromosome 2B. The TaAFP-D fragment (ca. 550 bp) was also not detected with TaAFP-3LP and TaAFP-5RP specific to TaAFP-D in ditelo 2DL, which lacks the short arm of chromosome 2D. Therefore, TaAFP-D appears to be located on the short arm of chromosome 2D. Because of the unavailability of ditelo 2AL, we could not examine the chromosomal location of TaAFP-A. However, TaAFP-A appears to be located on chromosome 2A because the AFP gene (AB360914) isolated from T. urartu, an A genome donor species of wheat, showed higher similarity to TaAFP-A than to TaAFP-B and -D (data not shown).
 View Details | Fig. 3 Estimation of copy number of TaAFP genes by Southern blotting and their chromosomal location by amplification TaAFPs in ditelocentric lines. A; Southern blotting of TaAFP genes using a probe amplified with TaAFP-LP and TaAFP-RP primers. CS, normal wheat line Chinese Spring; nulli2B-tetra2A, a CS line with no chromosome 2B and 4 doses of chromosome 2A; nulli2D-tetra2A, a CS line with no chromosome 2D and 4 doses of chromosome 2A. B; Amplification of TaAFP-B and -D in ditelo2BL and ditelo2DL lines, which lack the short arm of chromosome 2B and 2D, respectively. A 750-bp fragment of TaAFP-B was amplified with a pair of primers, TaAFP-4LP (specific for TaAFP-B) and TaAFP-4RP. A 550-bp fragment of TaAFP-D was amplified with a pair of primers, TaAFP-3LP (specific for TaAFP-D) and TaAFP-5RP. |
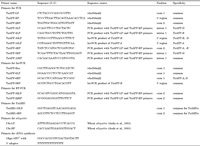
To narrow down the location of TaAFPs on the short arms, we used the deletion lines, which lack a part of the short or long arm of chromosomes 2A, 2B, and 2D. The fraction length of each line showed a relative fraction of the remaining arm and was obtained from Endo and Gill (1996) and Dr. T. R. Endo (Laboratory of Plant Genetics, Graduate School of Agriculture, Kyoto University, Japan).
Amplification of TaAFP-A was examined with TaAFP-14LP and -13RP primers in the deletion lines of 2AS-05, 2AS-08, and 2AL-02 (Fig. 4). The 2AL-02 line lacked the long arm of chromosome 2A. TaAFP-A was amplified in all the lines examined and appears to be located between the centromere of chromosome 2A and FL 0.59 on the short arm of chromosome 2A. TaAFP-B was amplified in the 2BS-01 (FL 0.53) but not in the 2BS-10 (FL 0.16) and 2BS-11 (FL 0.27) lines (Fig. 4). TaAFP-B appears to be located between FL 0.27 and FL 0.53 on the short arm of chromosome 2B. Because TaAFP-D was not amplified in the 2DS-05 line (FL 0.47), it appears to be located between FL 0.47 and the end of the short arm of chromosome 2D (Fig. 4).
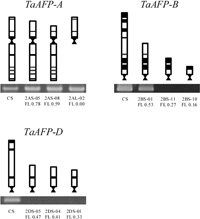 View Details | Fig. 4 Amplification of TaAFPs in the partial deletion lines of chromosome arm 2AS, 2AL, 2BS, and 2DS of wheat. TaAFP-A was amplified with TaAFP-14LP (specific for TaAFP-A) and TaAFP-13RP primers; TaAFP-B, with TaAFP-4LP (specific for TaAFP-B) and TaAFP-4RP primers; and TaAFP-D, with TaAFP-3LP (specific for TaAFP-D) and TaAFP-5RP primers. The primer sequences are listed in Table 2. |
About 800 bp of the 5’ region upstream from the start codon ATG of TaAFPs are relatively well conserved (Fig. 5). Transcription factor binding elements appear to be included in the 5’ region and were searched for using the Motif program (http://motif.genome.jp/). The ABA response element (ABRE, 5’-ACACGTGGC-3’) (Guiltinan et al., 1990) and its core element, G-box (5’-ACGT-3’), were found twice in all TaAFP genes (Fig. 5). A dehydration responsive element/C-repeat core sequence (DRE/CRT, 5’-CCGAC-3’) for responses to dehydration and low temperature stresses (Baker et al., 1994; Jiang et al., 1996) was also found in all of the 5’ regions. Possibly, TaAFP expression is affected by these types of stimuli (ABA, dehydration, and low temperature). The following elements were also found in all TaAFP genes: the CACA element, which both positively and negatively regulates the expression of storage protein (Chandrasekharan et al., 2003); the AACAA element, which is a binding site of the Myb-type transcription factor and is involved in the endosperm-specific expression of glutelin protein (Takaiwa et al., 1996); the GAmyb binding site (5’-YAACSGMC-3’) (Gubler et al., 1995) and the P (Myb-type) binding site (5’-ACCWACCNN-3’) (Grotewold et al., 1994). The following elements were found in one or two of the TaAFP genes. The RAV1 (related to ABI3/VP1, B3 domain-type) transcription factor requires bipartite elements (5’-CAACA-3’ and 5’-CACCTG-3’) (Kagaya et al., 1999). TaAFP-A and -D have these elements, whereas TaAFP-B has only the 5’-CACCTG-3’ element (Fig. 5). However, only TaAFP-B has a core element (5’-AAAG-3’) for the DNA binding with one finger (Dof) factor (Yanagisawa and Schmidt, 1999).
 View Details | Fig. 5 Transcription factor binding elements in the 5’ regions of the TaAFPs (TaAFP-A, TaAFP-B, and TaAFP-D). The start codon (ATG) is shown at the end of the sequences. The sites in boxes represent the transcription factor binding sites. ABRE, ABA response element (Guiltinan et al., 1990); G box, ACGT core element; CACA, element of several storage protein genes (Chandrasekharan et al., 2003); AACAA, element of endosperm-specific gene expression (Takaiwa et al., 1996); Dof, element for one finger type of transcription factor (Yanagisawa and Schmidt, 1999); RAV1, element for Arabidopsis cold-responsive transcription factor RAV1 (Kagaya et al., 1999); P, element for Myb-type transcription factor P (Grotewold et al., 1994); GAmyb: element for GA-regulated Myb factor (Gubler et al., 1995); DRE/CRT (Dehydration Responsive Element/C-repeat), element for cold and dehydration response (Baker et al., 1994; Jiang et al., 1996). |
The expression of the TaAFP genes (TaAFP-A, -B and -D) were estimated based on the size of the fragments (69-bp TaAFP-A, 79-bp TaAFP-B, and 57-bp TaAFP-D), which were amplified with TaAFP-32LP and TaAFP-22RP primers designed in the flanking region of the deletions in exon 2 (Fig. 6A). RNA was extracted from roots and leaves of the seedlings at day 7 after germination, flag leaves, grains at 10 DAP and embryos at 30 and 50 DAP. TaAFP-B was expressed predominantly in all tissues (Fig. 6B). TaAFP-D expression increased during grain development and showed tissue specificity. The effects of ABA and abiotic stresses, such as salt and dehydration, were evaluated (Fig. 6C). The expression of TaAFP-A and -D increased in roots treated with ABA (10–4 M), NaCl (200 mM) or dehydration (0.3% reduction of water content) for 1 h. When the same treatments were tested for longer duration periods (3, 6, and 12 h), the expression of TaAFP-A and -D reached the same level observed in the 1-h treatment.
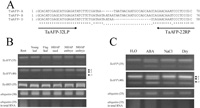 View Details | Fig. 6 Expression of TaAFPs and TaABI5s. A; Primer positions for RT-PCR in exon 2 of TaAFPs. Three different size fragments (69-bp TaAFP-A, 79-bp TaAFP-B, and 57-bp TaAFP-D) were amplified with TaAFP-32LP and TaAFP-22RP primers. B; Expression of TaAFPs and TaABI5s in roots and leaves of the seedlings at day 7 after germination, flag leaves, developing grains at 10 DAP, and embryos of the grains at 30 and 50 DAP. TaAFP genes were amplified with TaAFP-32LP and TaAFP-22RP primers designed in the flanking regions of the insertions/deletions, and TaABI5s were amplified with TaABI5-19LP and TaABI5-4RP primers (Table 2). The PCR cycle number is shown in parentheses at the left side of the panel. C; Expression of TaAFP genes in seedling roots treated with ABA (10–4 M), NaCl (200 mM), or dehydration (incubated in a desiccation chamber [0.3% reduction of water content]) in the dark at 25°C for 1 h. TaAFP genes were amplified with TaAFP-32LP and TaAFP-22RP primers. The PCR cycle number is shown in parentheses at the left side of the panel. |
Three deduced amino acid sequences of TaAFPs were quite similar, although there were minor insertions/deletions among them (Fig. 2). TaAFPs have a nuclear localization domain in the middle and an ABI5 binding domain in the C-terminal regions. AtAFP (AAF67775) also has these domains in the middle and the C-terminal regions. A comparison of the base sequences of TaAFPs with those of the other genes (NP 189598, NP 566163, and AAL07175) of the subfamily of AtAFP revealed that TaAFPs are the most similar to AtAFP. Thus, TaAFPs appear to be homologues of AtAFP.
TaAFPs appear to be localized between FL 0.47 and FL 0.53 on the short arms of the chromosomes of homoeologous group 2 (Fig. 4). Anderson et al. (1993) reported a marker gene (xcdo064) associated with grain dormancy on the short arms of the same chromosomes. Xcdo064 was known to be localized between FL 0.53 and FL 0.75 on the short arms of homoeologous group 2 (Delaney et al. 1995). TaAFP might be a candidate gene for the QTL of grain dormancy.
Lopez-Molina et al. (2003) reported that AtAFP is mainly expressed in the early stage of germination and during seed development, at which time AtABA5 is also expressed. In the present study, TaAFPs, especially TaAFP-B, were activated in additional tissues, such as roots and leaves of seedlings and flag leaves (Fig. 6B). A combination of transcription factors in the 5’ region is considered to play an important role in tissue-specific expression. The promoter region of AtAFP has two G boxes for the bZIP-type factor (Guiltinan et al., 1990) and an AACAA box for the Myb-type factor, which are responsible for endosperm-specific expression (Takaiwa et al., 1996). However, Wu et al. (2000) showed that a GCN4 element (5’-A/GTGAC/GTCAT-3’) for the bZIP- type factor is a critical factor in endosperm-specific expression, and that the G-box and AACAA elements are involved only in the level of expression. AtAFP has a GCN4-like element in its 5’ region, whereas TaAFPs do not (Fig. 5). The absence of the GCN4 binding site in the 5’ region of TaAFPs may be responsible for the expression of TaAFP in a wider range of tissue types, such as roots and leaves.
A comparison of the expression levels of the TaAFPs revealed that TaAFP-B is expressed predominantly in all tissues. TaAFP-B has unique elements in the 5’ region, one Dof element, and an incomplete bipartite element required for RAV1 binding. Yanagisawa (2004) suggests that a Dof factor is expressed in the leaves, stems, and roots of both monocots and dicots, and that it is involved in many aspects of plant growth and development. If RAV1 does not function as a repressor, a Dof element of the TaAFP-B may be considered the factor responsible for its predominant expression.
The results demonstrate that TaAFP-D expression increases during grain development (Fig. 6B). We cannot find a reasonable element responsible for its expression pattern during grain development.
ABA, NaCl, and dehydration stresses induced TaAFP-A and TaAFP-D in all tissues examined (Fig. 6C). The transcription factors binding to ABRE (5’-ACACGTGGC-3’) for the ABA response and to DRE/CRT (5’-CCGA-3’) for the dehydration and low temperature responses do not appear to be primary factors for the upregulation of TaAFP-A and TaAFP-D because these elements were found in all TaAFPs. The RAV1 transcription factor is known to be involved in the plant responses to ABA, jasmonic acid, osmotic stress and wounding (Sohn et al., 2006). A bipartite element (5’-CAACA-3’ and 5’-CACCTG-3’) necessary for RAV1 binding (Kagaya et al., 1999) was found in TaAFP-A and TaAFP-D. The RAV1 factor (B3 domain-type) may be a factor that affects the TaAFP-A and TaAFP-D response to ABA, NaCl and dehydration stresses. These results also suggest that these redundant TaAFPs may differentiate their roles in ABA signaling during wheat evolution.
AtAFP was expressed only in the developing seeds and in the early stage of germination, at which time AtABI5 was expressed (Lopez-Molina et al., 2003). In comparison, TaAFPs, especially TaAFP-B, were activated in roots, leaves, and developing grains. We isolated eight wheat homologues (TaABI5) of AtABI5 (unpublished), one of which is the same gene of TaABF (Johnson et al., 2002). TaABI5s were expressed differently; some were activated only in developing grain, the others were in roots and leaves. We used the primers common for all TaABI5s isolated and detected the expression of TaABI5 as a whole. TaABI5s were expressed in those tissues where TaAFP expression was activated (Fig. 6B). In the ABA signaling pathway of wheat, TaABI5s (positive regulators) and TaAFPs (negative regulators) appear to function in a greater variety of tissues than in Arabidopsis.
This work was supported in part by a grant from the Ohara Foundation for Agricultural Research. We also thank the National Bioresource Project-Wheat (NBRP-KOMUGI) for providing wheat materials and the EST databases.
|