| Edited by Yoshibumi Komeda. Zhiying Ma: Corresponding author. E-mail: mzhy@hebau.edu.cn |
Glutathione S-transferases (GSTs) are cytosolic dimeric proteins with multi-physiological roles, which can catalyze the conjugation of the thiol group of glutathione (GSH) with far-ranging xenobiotic alkylating agents, hydrophobic substrates, electrophilic substrates and endogenous agents (Zettl et al., 1994; Jepson et al., 1997; Dixon et al., 1998; Koeppe et al., 1998). In general, the molecular mass of each subunit was in the range of 25–27 kDa in GSTs (Edwards et al., 2000). The expression of GSTs could be induced by herbicides, hormones, pathogen, environmental toxins, therapeutic drugs and products of oxidative stresses (Droog, 1997). Consequently, the GSTs could protect tissues against oxidative damages, reduce the organic hydroperoxides and transport amphiphatic compounds and plant hormones as carrier proteins (Marrs, 1996; Listowski et al., 1988).
In mammalian, six classes (alpha, mu, sigma, pi, theta and zeta) of GSTs were categorized based on substrate specificity, amino acid identity and immunocrossreactivity (Reinemer et al., 1996; Neuefeind et al., 1997a, 1997b). Initially, three types of plant GSTs, namely Type I, Type II and Type III, were recognized based on 16 full-length GST subunit sequence and the different number of exons (Droog et al., 1995). Subsequently, the nomenclature of animal GSTs was proposed to be extended and used in plant. And three classes of GSTs were set up, namely theta, tau and zeta (Droog, 1997). This classification system was amended following the full genome of Arabidopsis sequenced. Forty-eight GST-like genes were grouped into five classes, namely tau, phi, theta, zeta and lambda, based on deduced amino acid sequence (David et al., 2002).
In plants, a GST activity was firstly reported with detoxifying atrazine by catalyzing the formation of an atrazine-glutathione conjugate in maize (Shimabukuro et al., 1971). GSTs also played a role in targeting numerous secondary metabolites that may be phytotoxic to an appropriate cellular localization (Marrs, 1996). The tau and phi GSTs were specific in plant with the function of detoxifying atrazine, especially in detoxifying diphenylether and aryloxyphenoxyropionate herbicides. At the same time, in the tau and phi GSTs, only the subunits from the same class would dimerize. Moreover, the subunits within a class could dimerize even if they were quite different in amino-acid sequence (Dixon et al., 1999; Sommer and Böger, 1999). The synthesis of subunits, especially, could be induced under stress treatment (Dixon et al., 1997; Cummins et al., 1997). Interestingly, a tau GST from tomato could suppress apoptosis induced by the Bax protein in yeast (Kampranis, 2000). The tau class could also play a pivotal role in the signal transduction and the synthesis of anthocyanins (Marrs et al., 1995; Droog, 1997; Edwards et al., 2000). The tau GST subfamily had undergone extensive gene duplication in plant. Twenty-eight and forty tau genes were found in the genome of Arabidopsis and Oryza sativa, respectively, through analyzing the genome sequence (http://www.arabidopsis.org/; http://www.tigr.org/tdb/e2k1/osa1/index.shtml; http://www.ncbi.nlm.nih.gov/).
A so-called cotton GST cDNA fragment without HindIII site in the translation region was transferred to tobacco, and the transgenic plants showed the enhanced resistance to methyl viologen (Yu et al., 2003), but no more information of the gene sequence and amino acids were reported. In present study, we cloned a tau Glutathione S-transferase subunit encoding gene from cotton (Gossypium hirsutum). The nucleotide, protein, EST Blast at NCBI (http://www.ncbi.nlm.nih.gov/) and cotton ESTs alignment at AGI (http://www.genome.arizona.edu/est/cotton) showed that the GST was a new tau GST subunit encoding gene and named GhGST. The structure, mRNA expression and prokaryotic expression of the gene were analyzed.
Total RNA extraction was performed from the root of resistant upland cotton cultivar, Jimian20, using a modified CTAB-LiCl precipitation (Ambion, USA) at 8 h, 12 h, 24 h and 48 h after inoculating Verticillium dahliae, respectively. The mRNA was purified from total RNA using the PolyATract mRNA Isolation Systems according to the manufacturer’s instruction (Promega, USA).
A cDNA library including 800 positive clones was obtained by suppression subtractive hybridization (SSH) via the BD PCR-selectTM cDNA Subtraction Kit (Clontech, USA). The SSH protocol was as follows: mRNA from the root induced by Verticillium dahliae as a tester, mRNA of non-induced control as a driver, respectively. The double-strand cDNA was synthesized from 2 μg mRNA of tester and driver materials, respectively. Then the restriction digestion was operated with RsaI. The digested blunt ends of the tester cDNA were divided into two parts and ligated with adaptors 1 and 2. Following, two rounds of hybridization and two rounds of PCR were operated. The products from the secondary PCR were ligated into a pGEM-T Easy Vector using a pGEM-T Easy PCR Cloning Kit (Tiangen, Beijing). Positive clones were identified through digesting with EcoRI and nestsed PCR. Two hundreds and thirty-four positive cloning fragments were sequenced by Sangon, China. A 751 bp fragment homologous to GST was obtained. A pair of gene specific primers (GSPs) was designed for the 5’- and 3’- RACE reactions by Primer Premier5.0 and Oligo6 softwares. 5’- RACE and 3’-RACE GSP primers were G5 (5’-GTGACAGGCGTTCATTGGGAGG-3’) and G3 (5’-TGGCAACAAGAGCGATTTACT-3’), respectively. 5’- RACE and 3’- RACE were carried out using SMART RACE cDNA Amplification Kit (Clontech, USA). The PCR products were analyzed on a 1% agarose EtBr gel. The purified DNA was cloned into pGEM-T Easy Vector and transformed into E. coli Top10 according to the Kit manual instruction (Tiangen, China). Selected positive clones were analyzed using EcoRI digestion and PCR, then sequenced by Sangon, China. The sequenced results of 5’- RACE and 3’- RACE products were analyzed and aligned using DNAStar, DNAMAN software, NCBI blast and ProFunc blast (http://www.ebi.ac.uk/thornton-srv/databases/ProFunc/).
Total RNA samples (3 μg per reaction) from different tissues of root, stem, leaf and root induced by Verticillium dahliae were reversely transcribed into cDNA using the PrimeScript 1st Strand cDNA Synthesis Kit (TaKaRa, China). The cDNA fragments were amplified with the GSP primers G5 and G3 for a 589 bp fragment of GhGST. The cotton polyubiquitin gene (GhUBI; 5’-CTGAATCTTCGCTTTCACGTTATC-3’, 5’-GGGATGCAAATCTTCGTGAAAAC-3’, Li et al., 2005.) and actin gene (5’-CTTTCCCTCGCTTTCTCG-3’, 5’-AATTGGCACGGCTCCTTA-3’) were used as internal controls to normalize the differences of template concentrations. Thermal cycling was performed under the following conditions: one cycle at 94°C (5 min); then 25 cycles at 94°C (1 min), 58–60°C (1 min, the annealing temperature varied according to the sequence of the primers), and 72°C (1 min), followed by 72°C (10 min) for the final extension. PCR products were detected on 1% agarose gel and viewed under the Bio-RAD Gel Doc imaging devices.
The open reading frame (ORF) of GhGST with SacI and BamHI restriction sites was cloned into the expression vector pET-32A. The recombinant plasmid and pET-32A without GhGST gene were expressed in the E. coli BL21 (DE3). Protein expression was induced using IPTG for 3 h according to the pET system manual, and the protein extracts were analyzed using SDS-PAGE electrophoresis. The GhGST activity was assayed based on the GST catalyzed reaction between GSH and 1-chloro-2,4-dinitrobenzene (CDNB), and the produces were detected at A340 using spectrophotometer (Habig, 1974; Simons and Vander Jagt, 1977). Two milliliter of culture bacteria was collected by centrifugation containing GhGST-fusion protein and the protein of pET-32A without GhGST gene, respectively. The collected bacteria were broken by ultrasonic in 500 μl phosphate buffer (100 mM, pH 6.5), then centrifuged at 10,000 × g for 15 min at 4°C. The supernatants were used for the GhGST activity assay. The assay mixture contained 0.1 ml of 75 mM GSH (Sigma), 0.1 ml of 30 mM CDNB (Sigma), 0.1 ml of crude bacterial sonicates and 2.7 ml of 100 mM phosphate buffer (pH 6.5). The control contained 2.8 ml phosphate buffer and no crude bacterial sonicates. The absorbance was read once every minute at 340 nm for 5 min. Protein contents were determined according to lowry-kalckar formula: protein concentration (mg/ml) = 1.45 × A280–0.74 × A260 (An et al., 2001). One unit of GST activity was defined as 1.0 μM conjugation of CDNB with GSH per min at pH 6.5 at 25°C.
An obtained cDNA sequence was 919 bp, representing full length GhGST of Gossypium hirsutum. The ORF of the cloned GhGST was 678 bp with a protein translation start sites ATG present at the 39th base pairs. A termination codon TAA was present at the 716th base pairs. A HindIII site was present at the 419th base pairs, and a putative polyadenylation signal AATAAA at the 849th base pairs as well (Fig. 1).
 View Details | Fig. 1 The full-length cDNA sequence and the deductive amino acids sequence of GhGST (GenBank accession No. EU074792). The stop codon was represented by an asterisk. The putative polyadenylation signal was shown as boxed sequence in the 3’UTR. |
The GhGST was 225 amino acids length with molecular mass of 25.821 kDa calculated based on the sequence. rpsBLAST analysis showed that the GhGST belonged to tau subfamily and had two conserved domains, namely, GST_N_Tau and GST_C_Tau. Simultaneously, some strongly conserved sequences of the class tau were found in GhGST as found in previously reported other tau GSTs (Droog, 1997). The first conserved triplet of amino acid residues was present at position 52–54, histidine-lysine-lysine. The second conserved histidine-asparagine-glycine triplet was present at 60–62 in GhGST (Fig. 2). Moreover, the hydropathy analysis of the GhGST amino acid sequence showed that one main stretch of hydrophobic residues was present at position 149–166 of polypeptide. The polypeptide had one putative helix in the N-terminal domain and six putative helices in the C-terminal domain (data not shown).
 View Details | Fig. 2 Multiple sequence alignment of the deduced amino acid sequence of GhGST polypeptide with the polypeptides sequences of Arabidopsis thaliana (Accession No. NP_187538), Glycine max (AAG34801), Glycine max (AAG34803), Vitis vinifera (CAN63896), Lycopersicon (AAG16756) and Gossypium hirsutum (AF159229). The black indicates single fully conserved residues; the carnation and celeste indicates amino acids with strongly conserved groups. The two conserved triplets of amino acid residues were shown as boxed sequence in GhGST. |
The mRNA expression of GhGST was determined by semi-quantitative RT-PCR. The results showed that the mRNA expression of GhGST increased obviously under Verticillium dahliae stress in root in comparison with the non-inoculated control (Fig. 3A, 3B). All tissues contained GhGST mRNA. However, higher level of GhGST mRNA was detected in root than in stem and leaf. This revealed that GhGST was constitutive expression gene in cotton, and the expression levels varied in trophic tissues. (Fig. 3C).
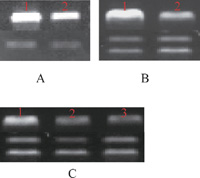 View Details | Fig. 3 Detection of mRNA expression of GhGST by semi-quantitative RT-PCR. (A) RT-PCR analysis of GhGST under Verticillium dahliae stress. Lane 1: root stress with Verticillium dahliae. Lane 2: root without stress by Verticillium dahliae, RT-PCR products of the cotton actin gene were used as internal control. (B) RT-PCR analysis of GhGST under Verticillium dahliae stress. Lane 1: root stress with Verticillium dahliae. Lane 2: root without stress by Verticillium dahliae, RT-PCR products of the cotton polyubiquitin gene (GhUBI) were used as internal control. (C) Lane 1, lane 2 and lane 3 were the RT-PCR products of root, stem and leaf without Verticillium dahliae stress. RT-PCR products of the cotton polyubiquitin gene (GhUBI) were used as internal control. |
The E. coli cell with pET-GhGST could be induced by IPTG, and a novel polypeptide with the molecular mass of about 45 kDa was expressed in E. coli by SDS-PAGE. The induced expression products of three unattached clones were coincident. The novel polypeptide contained GhGST protein (about 25kDa) and pET32A Tag fusion proteins (20.4kDa). But under the same culture conditions, the novel polypeptide could not be expressed in the control (Fig. 4).
 View Details | Fig. 4 The SDS-PAGE map of GhGST protein expression. M: protein marker MP102 (Tiangen, China). 1: pET32A vector induced with IPTG. 2–4: GhGST recombination protein containing His•Tag and S•Tag fusion proteins induced with IPTG. 5: pET32A vector not induced with IPTG. 6–8: GhGST recombination protein containing His•Tag and S•Tag fusion proteins not induced with IPTG. |
GhGST activity assay showed that the crude bacterial sonicates with pET-GhGST gene had high activity to CDNB substrate, and the activity values of two unattached clones with pET-GhGST induced by IPTG were 262.1 ± 10.7 and 280.82 ± 26.95, respectively. The results also implied that the GhGST subunit could dimerize. But the fact that the pET-32A without GhGST gene induced by IPTG had low activity to CDNB was also detected with the value 2.12 ± 1.77.
In the present study, we cloned and analyzed the tau class GST subunit encoding gene, GhGST, from Gossypium hirsutum under Verticillium dahliae stress in root. The ORF of the GhGST was 678 bp length, which encoded a 225 amino acids polypeptide. rpsBLAST analysis showed that GhGST had GST_N_Tau and GST_C_Tau conserved domains. The inducibility of tau GSTs under the abiotic and biotic stresses was a characteristic feature for tau GSTs genes (Marrs, 1996; Droog, 1997; David et al., 2002). Semi-quantitative RT-PCR indicated that the GhGST expressed in root, stem and leaf. But the expression of GhGST increased under Verticillium dahliae stress in root. Prokaryotic expression and activity assay of the GhGST showed that the GhGST could be induced by IPTG in E. coli BL21, and the GhGST had high activity to CDNB substrate.
The GhGST in this study was different from the previously reported GST cDNA fragment expressed in tobacco (Yu et al., 2003). Although any sequence information was not mentioned, the GST cDNA fragment cloned into pET28A with the HindIII site indicated that there was no HindIII site in encoding region of the GST cDNA fragment. However, the ORF of GhGST had the HindIII site in the 419th bp with the AAGCTT sequence.
Semi-quantitative RT-PCR analysis showed that the mRNA of GhGST was a constitutive expression gene in cotton trophic tissues. But, the content of GhGST expression increased obviously under Verticillium dahliae stress in root. Moreover, the PCR results were similar when GhUBI and actin gene were used as internal control. The result was similar to previously reported that GSTs expression was regulated predominantly at the level of transcription (Marrs, 1996; Davies and Caseley, 1999).
The transcription regulation of individual subunits ultimately influenced the range of GSTs heterodimers and homodimers formed (Cummins et al., 1997; Dixon et al., 1997). For example, three tau GST subunits, TaGSTU2, TaGSTU3 and TaGSTU4, were induced following safeners stress in wheat, and resulted in their dimerization with the TaGSTU1 subunit to form the TaGSTU1–2, TaGSTU1–3 and TaGSTU1–4 heterodimers, respectively (Cummins et al., 1997). In this study, the GhGST protein had high activity to substrate CDNB, indicating that the GhGST could dimerize and had biological function. Simultaneously, the crude pET-32A without GhGST gene protein had low GST activity, similar to that previously reported (Yu et al., 2003).
A distance tree with 100 alignments was obtained through BlastP with putative amino acid sequence. Five sequences, Arabidopsis thaliana (Accession No. NP_187538), Glycine max (AAG34801), Glycine max (AAG34803), Vitis vinifera (CAN63896) and Lycopersicon (AAG16756), had the most contiguous distance with the GhGST in the tree (data not shown). The above six sequences were aligned with a deduced amino acid sequence of the only published cotton GST complete cds using DNAMAN analysis (AF159229). The results indicated that percentage identities shared by GhGST with other GST proteins of Vitis vinifera (CAN63893), Glycine max (AAG34803), Arabidopsis thaliana (NP_187538), Lycopersicon (AAG16756), Glycine max (AAG34801) and Gossypium hirsutum (AF159229) were 55.56, 54.59, 51.75, 48.46, 44.3 and 30.14%, respectively. Sequence analysis of these seven GSTs revealed a highly conserved N-terminal domain in contrast to a highly variable C-terminal domain. The phylogenetic tree showed that GhGST and AF159229 were classed into the same group (Fig. 5).
 View Details | Fig. 5 Phylogenetic tree of the deduced amino acid sequence of the GhGST and the amino acid sequences of Arabidopsis thaliana (Accession No. NP_187538), Glycine max (AAG34801), Glycine max (AAG34803), Vitis vinifera (CAN63896), Lycopersicon (AAG16756) and Gossypium hirsutum (AF159229). The phylogenetic tree was inferred using DNAMAN software. The distances from the nodes, i.e. the branch lengths denoted in the tree, correspond to sequence divergence. |
The biochemically functional prediction of GhGST protein was done by using ProFunc. The result showed that the GhGST protein had the most similar function to the tau class glutathione S-transferase from wheat (PDB code: 1gwc; PubMed id: 12033934), which had the activity of herbicide detoxification (Thom et al., 2002).
As all stated above, the GhGST was a new tau GST subunit encoding gene and the first cloned GST subunit encoding gene under Verticillium dahliae stress in Gossypium hirsutum.
We thank Dr. Abdul Razzaq of the University of Arid Agriculture, Pakistan for his critical reading of the manuscript. This research was supported by the Early-Stage Basic Research Key Project of China (No. 2004CCA01100); the Natural Science Foundation of China (No. 30471105) and the Natural Science Foundation of Hebei province (No. C2004000365; C2006001034).
|