| Edited by Kiichi Fukui. Masao Watanabe: Corresponding author. E-mail: nabe@ige.tohoku.ac.jp. Sachiyo Isokawa and Masaaki Osaka: They are equally contributed to this work. Nucleotide sequence data; Nucleotide sequence data are available in the GenBank database under the accession numbers AB543254 for Sx-SP11 and AB543255 for Sz-SP11. |
Self-incompatibility (SI) is defined as the inability of a fertile hermaphrodite plant to produce zygotes after self-pollination (de Nettancourt, 2001). In Brassicaceae, selfed pollen is rejected at the surface of papilla cells on the stigma, owing to the recognition of self versus non-self pollen (Iwano et al., 2003). The SI system in Brassicaceae is sporophytically controlled by a single locus, termed S, with multiple alleles (Bateman, 1955). In a recent analysis, male and female S determinants located at the S-locus were identified and characterized (reviewed in Watanabe et al., 2003, 2008; Takayama and Isogai, 2005). The male S determinant, termed SP11/SCR (S-locus protein 11/S-locus cysteine-rich protein), encodes a small cysteine-rich protein and is a member of defensin-like pollen-coat proteins (Suzuki et al., 1999; Schopher et al., 1999; Takayama et al., 2000; Shiba et al., 2001). The female S determinant, SRK (S-locus receptor kinase), functions at a surface of stigma papilla cells as a receptor for SP11 (Takasaki et al., 2000). The SP11 and SRK physically interact in an allele-specific manner (Takayama et al., 2001), and this triggers the following downstream signaling cascade for the SI reaction. Although a few factors of the signaling cascade have been identified and characterized (Stone et al., 1999, 2003; Murase et al., 2004; Samuel et al., 2008; Ivanov and Gaude, 2009), a comprehensive understanding of the whole SI mechanism still remains unexplained in Brassicaceae.
Self-compatible (SC) mutants are significant resources for identifying factors on the SI system, because SC has been reported to be mainly caused by a disruption of S-locus genes and genes involved in the following signaling cascade. Several lines showing SC have been isolated and characterized (reviewed in Hinata et al., 1993; Watanabe and Hinata, 1999; Takayama and Isogai, 2005), and these lines are basically classified into two groups. The first group is categorized as a disruption of the S-locus genes, SP11 and/or SRK. For example, a self-compatible B. napus had a 1-bp deletion at the end of the S domain on the SRK coding region, leading to a truncated and functionally defective SRK (Goring et al., 1993). In the other group, SC is owed to a disruption of the non-S-locus genes, i.e. the mutation in the factor(s) on a signaling cascade. In the latter group, one of the SC mutants (B. rapa var. yellow sarson), derived from India, had a recessive epistatic modifying gene (M), which functions in the stigma but not in the pollen (Hinata et al., 1983). It is known from map-based cloning that the M gene encodes a membrane-anchored cytoplasmic serine/threonine protein kinase MLPK (M-locus protein kinase), a positive mediator of Brassica SI signaling (Murase et al., 2004; Shimosato et al., 2007), and its protein product could directly interact with SRK (Kakita et al., 2007). In another case, the antisense ARC1 (arm repeat containing 1) transgenic plants showed partial SC (Gu et al., 1998; Stone et al., 1999). It has been reported that a putative ARC1 protein interacts physically with the kinase domain of SRK, and thus downstream of the SP11-SRK signaling cascade would be inhibited by a disruption of ARC1.
Although several SC lines had been identified as described above, in most cases their molecular and genetic analyses were not performed well (reviewed in Takayama and Isogai, 2005). During several decades, SI research of most plant species has focused mainly on the identification of recognition factors between pollen and the pistil, such as SP11 and SRK in Brassica. Furthermore, because most of the SC plant material had already been lost, it is impossible to perform further analysis of such lost material. For these reasons, a screening and establishment of novel SC lines are critical as genetic resources for SI research in Brassica and Brassicaceae.
Some SC lines have been identified from SI populations of Brassica and its relative species (Nasrallah and Wallace, 1968; Thompson and Taylor, 1971; Nou et al., 1991, 1993a, 1993b). From the viewpoint of genetic resources for SI research, we focused on screening SC lines from the bulk-populations of traditional and commercial vegetables of B. rapa, including a turnip, turnip green, Chinese cabbage, oilseed rape and other leafy vegetables. By a screening of variety of B. rapa vegetables, three SC lines termed as TSC (Talented Self-Compatible line), TSC2, TSC4, and TSC28, were isolated. In this study, we analyze their genetic and molecular characteristics, for the purpose of identifying pieces of the SI signaling pathway in Brassicaceae. We also discuss the utility of SC lines for analysis of SI in the Brassica species and genetic resources for plant breeding.
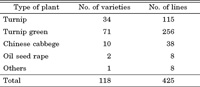
Seeds of 78 B. rapa lines were obtained from the following seed companies: Tohoku Seed Co. Ltd. (Utsunomiya, Japan), Kaneko Seed Co. Ltd. (Maebashi, Japan), and Watanabe Seed Co. Ltd. (Kogota, Japan). Seeds of another 40 lines were obtained as a generous donation from Kyoto Agricultural Experimental Station and Hiroshima Agricultural Experimental Station (Table 1). All of the commercial and traditional lines selected here are not F1 hybrids, but rather bulk-populations of mass seed production. Homozygous S-tester lines (S8 and S40) were maintained at Tohoku University (Nou et al., 1991). For linkage analysis, F2 populations were developed by crosses between the TSC4 and S8 tester line, and the TSC28 and S8 line. All plants in this study were grown in a greenhouse under normal conditions.
 View Details | Table 1 Plant variety used in this study |
For test pollinations, non-hand pollinated flowers were discarded at the peduncle. Self- and cross-pollinations were performed with emasculated flower buds, and pollinated flowers were placed on a 1% solid agar for 24 h under room conditions. Pistils of the pollinated flowers were stained with aniline blue solution (100 mM K3PO4, 0.1% aniline blue), as described by Hatakeyama et al. (1998). Picked pistils were mounted on a glass slide with 50% glycerol and observed by UV fluorescence microscopy (Eclipse E800 microscope system; Nikon, Tokyo, Japan). The degree of SI/SC phenotype in test-pollination was classified into the following six categories (0–5) based on the rate of pollen tube penetration into the stigma: (0) no pollen tube penetrated to the stigmatic papilla nor grew into a style, (1) 1–5 pollen tubes penetrated into a style, (2) 6–10 pollen tubes penetrated into a style, (3) 11–15 pollen tubes penetrated into a style, (4) 16–20 pollen tubes penetrated into a style, and (5) more than 21 pollen tubes penetrated into a style. In each test pollination, at least three flowers were used and replicated more than three times on different days.
To obtain SP11 partial nucleotide sequences from each line, reverse transcriptase (RT)-PCR was performed using universal primers for SP11; SP11-I-F and SP11-I-R-NotI-dT for class I, and SP11-II-F and SP11-II-R for class II (Table 2). Poly (A)+ RNAs isolated from anther tissue were reverse-transcribed to the first strand cDNA using a First-Strand cDNA synthesis kit (Roche, Basel, Switzerland) and used as a template for RT-PCR (Watanabe et al., 2000, Shiba et al., 2002). RT-PCR condition was as follows: initial denaturation at 95°C for 1 min, followed by 30 cycles at 95°C for 1 min, 50°C for 1 min, and 72°C for 1 min. PCR products were electrophoretically-fractionated in a 1% agalose gel, stained with ethidium bromide, and then viewed under a UV illuminator. After confirmation of successful amplification, PCR products were sequenced directly using an ABI PRISM 310 Genetic Analyzer (Applied Biosystems, CA, USA) and compared with reference SP11 nucleotide sequences using BLASTN. On the basis of partial nucleotide sequences, full length SP11 sequences were determined by using Ambion FirstChoice RLM-RACE kit (Applied Biosystems). Using the information, the S haplotype specific-reverse primers were also designed for each S haplotype (Table 2).
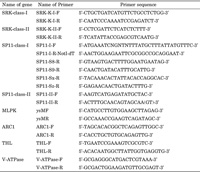 View Details | Table 2 Primer sequences for SI and SI-related genes used in this study |
Genomic DNA of each plant was extracted from young leaves as described by Murray and Thompson (1980).
For linkage analysis of the S haplotype and SI/SC phenotype, PCR was performed with a set of S haplotype specific SP11 primers: Sz and S8 for an F2 population of the TSC4 × S8 tester line, and Sx, S40 and S8 for the TSC28 × S8 tester line (Table 2). PCR amplification was performed with the following conditions: initial denaturation at 95°C for 1 min, followed by 30 cycles at 95°C for 1 min, 50°C for 1 min, 72°C for 1 min, and a final extension at 72°C for 4 min. PCR products were electrophoretically-fractionated in a 1% agalose gel, stained with ethidium bromide, and then viewed under a UV illuminator.
For genetic mapping of SI-related genes, PCR was performed under the same conditions mentioned above. Primer information for the SI-related genes is listed in Table 2.
RT-PCR was performed as described by Endo et al. (2004). To summarize briefly, poly (A)+ RNAs isolated from stigma and anther tissues were reverse-transcribed to the first strand cDNA using a First-Strand cDNA synthesis kit (Roche). After the RT reaction, cDNA was subjected to the following PCR with a set of specific primers for each gene (Table 2). PCR was performed with ExTaq DNA polymerase (TaKaRa BIO, Shiga, Japan) for 25 or 30 cycles of denaturation at 95°C for 1 min, annealing at the optimum temperature of each primer for 2 min, and extension at 72°C for 2 min. As a positive control, a gene encoding H+-vacuolar-ATPase (V-ATPase) specific primer was used (Takasaki et al., 2000). PCR products were electrophoresed in a 1% agalose gel, stained with ethidium bromide, and then viewed under a UV illuminator.
Mapping of the known SI related genes was conducted using an F2 population AG, consisting of 94 lines. The AG population was developed from a cross between Chinese cabbage parental line Nou 7 (A9709) and G004 at the Japanese National Institute of Vegetable and Tea Science (Suwabe et al., 2003). Genotyping of genes was carried out as described by Suwabe et al. (2008), and genes’ mapping onto the linkage map was performed using JoinMap 3.0 (Van Ooijen and Voorrips, 2001). The threshold for goodness of fit was set to ≤ 5.0, a recombination frequency of < 0.4, and minimum logarithm of odds (LOD) score of 3.0. All genetic distances are expressed in centimorgans as derived by the Kosambi mapping function (Kosambi, 1944). A previously published linkage map of the AG population (Suwabe et al., 2006, 2008) was used as a reference so that the position and order of each locus were confirmed. Primer information for mapping of the known SI-related genes is listed in Table 2.
For genetic mapping of the SC factors (genes) of TSC4 and TSC28 to a reference linkage map, SSR markers on the AG linkage map were used as anchors. The SSR markers which showed polymorphism among TSC4, TSC28, and S8 tester lines were selected for genetic analysis. The association between the genotype of each SSR locus in their F2 populations and the mean degree of SI/SC phenotype of each F2 individual was evaluated manually. The positions of SC factors on the linkage map were estimated using the MQM option of the MapQTL 5 software (Van Ooijen, 2004), with a LOD threshold of 3.65, and integrated into the reference linkage map.
In Japan, because most Brassica vegetables are F1 hybrid varieties, the parental lines having a rigid SI are essential to develop hybrids steadily. Such parental varieties produce offspring with rigid SI. In addition, because commercial F1 varieties are developed by several seed companies, the genetic origins and/or resources of parental lines are obscured in most cases. Therefore, it is difficult to find self-compatible lines from these hybrid varieties. Meanwhile, a few types of commercial Brassica vegetables are produced by methods of mass breeding, because market scale of such varieties is limited and there is no requirement for making a hybrid variety. As a result of mass breeding, they are not genetically pure and have a genetic variability in the population, meaning that they should contain lines having several levels of SI. Similarly, traditional varieties, which were preserved as genetic resources at national and prefectural experimental stations, are also maintained by bulk-population breeding. Thus, these varieties have the potential to have several levels of SI.
Based on these context and background of plant materials for breeding, we selected 118 varieties from traditional and commercial B. rapa vegetables for a screening of SC plant resources. In each variety, more than three plants were grown, and the degree of SI/SC phenotype was evaluated by test-pollination. In total, 425 lines of B. rapa vegetables were screened in this experiment. As explained in the MATERIALS AND METHODS section, we classified the SI/SC phenotype into six categories, and we regarded the lines as SI when the mean value of the SI/SC phenotype was smaller than 1.0. When the point of the SI/SC degree was between 1.0 and 3.0, the lines were classified into an intermediate phenotype. When the point was larger than 3.0, the lines were classified into SC. Of the 425 lines, 263, 131, and 31 lines were clearly classified as SI, intermediate, and SC, respectively (Table 3). The ratio of the SI/SC phenotype in the experiment is consistent with that in naturalized and wild B. rapa populations (Nou et al., 1991, 1993b).
 View Details | Table 3 Frequency of self-incompatible and self-compatible lines in the Japanese traditional and commerical vegetable populations in B. rapa |
From the 31 lines categorized as SC, three lines showing the stable SC phenotype, TSC2, TSC4, and TSC28, were selected (Fig. 1). The degree of SI/SC phenotypes in TSC2, TSC4, and TSC28, were 3.34, 3.56, and 3.22, respectively. Other 28 lines were also categorized as SC in test-pollination, however, they showed lower value of the SI/SC phenotype. Using these data, we conducted the following genetic and molecular analyses. First, in each line, the S haplotype was determined by a comparison of nucleotide sequences of SP11 in the public database (Fig. 2). In TSC4 and TSC28, novel S alleles, Sz and Sx, were found and, based on sequence similarity, both alleles were classified into class-I, the dominant S allele class for pollen phenotypes. Consequently, S haplotypes of TSC2, TSC4, and TSC28 were defined as S40S40, SzSz, and SxS40, respectively. Interestingly, out of three lines, the S40 allele was observed in two lines, although these lines were independent of each other in terms of vegetable variety and were collected from different geographic regions. S40 is classified as a class II S allele, which is genetically recessive against any other class I S alleles in pollen (Hatakeyama et al., 1998). This is uncommon, compared to the class I S allele; only 5 class II S alleles have been identified in B. rapa, whereas more than 80 S alleles have been identified in the Brassica species (Nou et al., 1993a; Ockendon, 2000). Because the genetic effect of the class II S allele is masked when makes a heterozygote with any class I S allele, it would be advantageous to maintain a species in a small or limited population, such as a mass breeding population. The SI system is initiated by the recognition reaction between pollen and stigma, and when both S haplotypes of pollen and stigma are identical, pollen is inhibited, leading to a failure of pollination and fertilization, i.e. a failure of biological reproduction. Thus, the class II S allele would act as a reproductive assurance and enhance the chance to produce progeny even if it had identical genetic characteristics and origins.
 View Details | Fig. 1 SI/SC phenotype on stigma papilla cell in the B. rapa lines. Pollen tube behavior on the stigma papilla was observed by basic aniline blue staining. In the case of SC lines, pollen tube penetrations were observed (A to C), as well as compatible pollination (D). In contrast, no pollen tubes penetrated the self-incompatible S9 stigma (E). A, TSC28; B, TSC2; C, TSC4; D, S9 tester line × S24 tester line; E, S9 tester line × S9 tester line. Arrow with Pc indicates papilla cell. Arrow with Pt shows pollen tube. Bar = 50 μm. |
 View Details | Fig. 2 Multiple alignment of predicted partial amino acid sequences of SP11 in B. rapa. Dashes represent gaps introduced to optimize alignment. Conserved residues, eight cysteine residures (C1 to C8), a glycine residure (#), and an aromatic amino acid residure (*) are boxed. |
In reciprocal test-cross pollination between the TSC2 (S40S40) and S40 tester line, when TSC2 was used as a female parent, the S40 tester pollen was compatible. In contrast, when TSC2 was used as a male parent, TSC2 pollen was incompatible on the S40 tester stigma. This result indicates that TSC2 has a female part mutation in the SI system. On an F2 population between the TSC2 and S8 tester line, pollination phenotypes segregated into SI:SC = 16:4 (3:1, χ2 = 0.606, P > 0.05), and the SC phenotype in the F2 progeny was only observed in S40 homozygous lines, whereas SI was observed in either line of S8S8 or S8S40 haplotypes. In the S8S40 heterozygous lines, because S40 is a class II S allele, the genetic effect of the S40 allele is masked by the S8 allele, and the genotype in pollen is expressed as S8. Thus, the SC phenotype was coincident with a segregation pattern of the S40 haplotype in the population. Taken together, TSC2 has a stigma-part mutation, and its SC phenotype is owed to a functional disruption in the S-locus, suggesting that TSC2 has a mutant of the female factor in the S-locus, i.e. SRK. This reconfirms that a disruption of the SI recognition factor(s) of stigma and/or pollen is critical for maintenance of the SI system and that our strategy is appropriate for screening of the SC mutants from the bulk-populations of traditional and commercial Brassica vegetables. Because our objective was to identify novel SC lines which have a mutation in the following cascade on the SI system, TSC2 was excluded from further analysis.
In TSC4 and TSC28, because Sz and Sx are novel S alleles and the genetic dominance relationship between other known S alleles is not available, a reciprocal test-cross analysis with a tester line cannot be performed. As another strategy for genetic analysis of TSC4 and TSC28, F2 populations were established by a cross with the S8 tester line. In F2 populations, we categorized pollination phenotype into 6 categories (0–5) and did the same with a test-pollination population, as mentioned above. We classified the populations into the following 2 groups: a mean value of the SI/SC phenotype of 0–1.9 (SI), and a mean value of the phenotype larger than 2.0 (SC). In both crosses, F1 progenies showed rigid and stable SI. In the F2 population of the TSC4 × S8 tester line, the pollination phenotype was segregated into SI:SC = 78:13 (6:1, χ2 = 1.0, P > 0.05) (Fig. 3A), and S haplotypes in the population were segregated into S8S8:S8Sz:SzSz = 12:47:32 (1:2:1, χ2 = 0.012, P < 0.05) (Fig. 4A). In this population, some factors are expected to be involved in the genetic effect for the SC phenotype. An intermediate SI/SC phenotype was also observed in some lines of the population. In the F2 population of the TSC28 × S8 tester line, the pollination phenotype was segregated into SI:SC = 67:27 (3:1, χ2 = 0.404, P > 0.05) (Fig. 3B). Because S alleles observed in this population were S8 and S40, but not Sx, the F1 line selected here was an S8S40 heterozygote. S haplotypes in the F2 population were segregated into S8S8:S8S40:S40S40 = 26:48:20 (1:2:1, χ2 = 0.667, P > 0.05). There was no correlation in segregation pattern between the S haplotype and the SI/SC phenotype. In other words, the S8-SI, S8-SC, S40-SI, and S40-SC plants were observed in TSC28 × S8 F2 progeny (Fig. 4B). For all of these results, it is suggested that at least two or more factors in the SI signaling pathway were mutated in these two lines.
 View Details | Fig. 3 Frequency distribution of the SI/SC phenotype in two F2 populations derived from TSC4 × S8 (A) and TSC28 × S8 (B). The degree of SI/SC phenotype in test-pollination was classified into the following six categories (0–5) based on the rate of pollen tube penetration into the stigma: (0) no pollen tube penetrated to the stigmatic papilla nor grew into a style, (1) 1–5 pollen tubes penetrated into a style, (2) 6–10 pollen tubes penetrated into a style, (3) 11–15 pollen tubes penetrated into a style, (4) 16–20 pollen tubes penetrated into a style, and (5) more than 21 pollen tubes penetrated into a style. |
 View Details | Fig. 4 Genetic linkage analysis between the S haplotype and the pollination phenotype. F2 progeny plants, derived from the TSC4 × S8 tester line (A) and the TSC28 × S8 tester line (B), were used to evaluate a genetic linkage between the S-locus and the pollination phenotype. For genotyping the S-locus, PCR was performed with SP11 gene-specific primers. The degree of SI/SC phenotypes was determined by self pollination in each line. S haplotypes in each line are represented as follows: Z, SzSz; 8, S8S8; 40, S40S40; H, SzS8 in A and S8S40 in B. |
In TSC4 and TSC28, gene expressions of the SI (SP11 and SRK) and SI-related (MLPK, ARC1, and THL) genes were investigated by RT-PCR. All five genes were expressed in both lines, and their expression levels were comparable with those in the SI tester line, S8 (Fig. 5). This result indicates that SI and SI-related genes, which are already identified and characterized, are expressed normally in TSC4 and TSC28. However, it is still unclear whether these factors function at the protein level and whether they are independent from a disruption of the SI system in TSC4 and TSC28. To resolve this, we compared positions of SI and SC factors on a genetic linkage map. If their positions are independent of each other, all known SI factors have no cause and effect relationship in a disruption of the SI system in TSC4 and TSC28, and the SC factors should be novel components of the SI system in Brassica. The putative SC genes of TSC4 and TSC28 were mapped on linkage groups A3 and A1, respectively. In TSC4, although some SC factors were expected, other factors could not be detected because of the resolution limit of the DNA markers used. Meanwhile, MLPK, ARC1, and THL were mapped on A3, A4, and A6, respectively (Fig. 6). Although potential SC genes of TSC4 and MLPK were on the same linkage group, their positions were apparently discriminated. It was also impossible to map the S-locus onto the linkage map because of an identical S haplotype, S60S60, in parental lines (data not shown), but, from the result of segregation analysis between the S haplotype and the SI/SC phenotype (Fig. 4), it is certain that SC genes of TSC4 and TSC28 have no correlation to the S-locus. These results clearly indicate that the SC genes of TSC4 and TSC28 are independent from the S-locus or known SI-related genes and that both lines are novel SC mutants. SP11 acts as a ligand of SRK and is sufficient to induce a SI reaction as a male S component (Takayama et al., 2000, 2001). Meanwhile, SRK is a protein kinase which triggers the phosphorylation of specific downstream proteins. Although the downstream signaling network of SRK is not understood completely, some factors should be involved in the network. Because the genes on the S-locus, i.e. SP11 and SRK, have no relation to the SC phenotype in TSC4 and TSC28, SC causal mutations in both lines would occur in the female-part downstream factors.
 View Details | Fig. 5 Expression analysis of SI and SI-related genes in TSC4, 28, and wild-type plants. Poly (A)+ RNA was extracted from the anther and stigma of TSC4, TSC28, and wild-type lines (S8 tester line). Reverse-transcribed cDNA in each sample was used for PCR amplification with a set of specific primers for each gene. The V-ATPase gene was used as a positive control. WT, wild-type. |
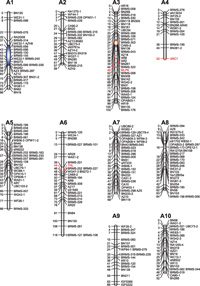 View Details | Fig. 6 Mapping of SC and SI-related genes on a reference linkage map. SC and SI-related genes were mapped on the reference linkage map of B. rapa (Suwabe et al., 2006; Suwabe et al., 2008). Positions of putative SC genes of TSC4 and 28 were represented as orange and blue ovals, respectively. Recombination distances are given in Kosambi centimorgans on the left side of the linkage groups, and the gene and locus names are given on the right side. The number inside the parentheses at the top of each linkage group indicates the internationally agreed chromosome nomenclature of B. rapa. |
In conclusion, the SC mutant lines selected from traditional and commercial Brassica vegetables of bulk population have mutations in the S-locus and novel factors of the SI system. These lines will contribute to the identification of pieces of the signal transduction pathway of the SI system in Brassica. They will also open new avenues for research into a comprehensive understanding of the SI mechanism in Brassicaceae.
The authors thank Professor Yoshibumi Komeda (The University of Tokyo) for his valuable advice. The authors are also grateful to Ayako Chiba (Iwate University), Kayoko Furukawa, Kazue Imataka, Tsukasa Kasahara, Nobuhide Kobayashi, Hiromi Masuko, Kosuke Matsumoto, and Masumi Miyano (Tohoku University) for technical assistance. We gratefully acknowledge Dr. Satoru Matsumoto (National Institute of Vegetable and Tea Science) for providing the B. rapa AG mapping population. This work was supported in part by Grants-in-Aid for Special Research on Priority Areas (Nos. 18075003, 18075012, 20380002, and 20678001 to M.W.), a Grant-in-Aid for Creative Scientific Research (No. 16G3016 to A.I., S.T., and M.W.) and a Grant-in-Aid for Young Scientists (Start-up) (No. 21880022 to K.S.) from the Japan Society for Promotion of Science (JSPS).
|