| Edited by Yusaku Nakabeppu. Qiu-Mei Zhang-Akiyama: Corresponding author. E-mail: qmzhang@kingyo.zool.kyoto-u.ac.jp. Shin-Ichiro Yonekura: Present address: Institute of Molecular and Cellular Biosciences, The University of Tokyo, Yayoi, Bunkyo-ku, Tokyo 113-0032, Japan |
Reactive oxygen species (ROS) are continually generated in living cells during normal cellular metabolism as well as by exposure to ionizing radiation and various chemical oxidizing agents. Cellular DNA and its precursor nucleotides are at high risk of being oxidized by ROS (Wallace, 2002; Cadet et al., 2003; Bjelland and Seeberg, 2003). 7, 8-dihydro-8-oxoguanine (8-oxodG) is one of the predominant base modifications produced by ROS at the C8 position of 2’-deoxyguanosine in DNA or on the free nucleotide form of guanine in the nucleotide pool (Sekiguchi and Tsuzuki, 2002; Bjelland and Seeberg, 2003). When generated in DNA, 8-oxodG directs insertion of dATP as well as dCTP opposite the lesion during DNA replication, which would generate G:C to T:A transversions (Hsu et al., 2004; Bjelland and Seeberg, 2003; David et al., 2007). 1, 2-dihydro-2-oxoadenine (2-oxodA) also has potent mutagenic activity. It pairs with dC and dG (Bjelland and Seeberg, 2003; Kamiya, 2004). Base excision repair (BER) is a critical mechanism for preventing mutations by removing the causative damaged bases from DNA (David et al., 2007; Hirano, 2008; Zharkov, 2008). DNA glycosylases hydrolyze the N-glycosylic bond between the oxidized base and deoxyribose, thus releasing a free base and an apurinic/apyrimidinic (AP) site in DNA. The resulting AP sites are further processed during BER (David et al., 2007; Hirano, 2008; Zharkov, 2008).
Oxidation of dG and dA also proceeds in the nucleotide pool, and the oxidized forms, 8-oxo-dGTP and 2-oxo-dATP, are potent mutagenic substrates for DNA replication. 8-oxo-dGTP is readily inserted by DNA polymerases opposite dA in a template, while 2-oxo-dATP is incorporated opposite dG and dC (Maki and Sekiguchi, 1992; Hayakawa et al., 1995; Sekiguchi and Tsuzuki, 2002; Bjelland and Seeberg, 2003). The incorporation of these oxidized nucleotides would generate base substitutions. Therefore, sanitization of oxidized nucleotide precursors is also an essential mechanism for suppressing base substitutions. In E. coli, MutT is involved in the prevention of mutations caused by 8-oxo-dGTP (Maki and Sekiguchi, 1992; Sekiguchi and Tsuzuki, 2002; Bjelland and Seeberg, 2003). MutT and its human homologue hMTH1 have pyrophosphohydrolase activity that hydrolyzes 8-oxo-dGTP to 8-oxo-dGMP and inorganic pyrophosphate (Maki and Sekiguchi, 1992; Sekiguchi and Tsuzuki, 2002; Xia et al., 2005). hMTH1 also has an activity that degrades 2-oxo-dATP (Sakai et al., 2002; Yoshimura et al., 2003; McLennan, 2006). E. coli Orf135 also has a pyrophosphohydrolase activity that hydrolyzes 2-oxo-dATP to 2-oxo-dAMP (Kamiya et al., 2003). In addition, MutT and human NUDT5 have 8-oxo-dGDPase activity that degrades 8-oxo-dGDP to 8-oxo-dGMP (Sekiguchi and Tsuzuki, 2002; Ishibashi et al., 2003; Kamiya et al., 2009). 8-oxo-dGMP and 2-oxo-dAMP are not rephosphorylated to the triphosphate form and therefore are not incorporated into DNA by DNA polymerases. On the other hand, 8-oxo-dGDP is phosphorylated by nucleoside diphosphate kinase to generate 8-oxo-dGTP, which can be incorporated into DNA (Sekiguchi and Tsuzuki, 2002; Ishibashi et al., 2003). Therefore, the 8-oxo-dGDPase activity also plays an important role in sanitization of the nucleotide pool to prevent mutations (Sekiguchi and Tsuzuki, 2002; Ishibashi et al., 2003). Almost all organisms examined so far possess such enzymes that hydrolyze their preferred oxidized nucleotides. These enzymes must play an important role in protection of the genome from oxidation by ROS.
The ascidian (sea squirt) Ciona intestinalis is a multicellular eukaryote that belongs to the subphylum Urochordata, the earliest branch in the phylum Chordata. Nucleotide sequencing of the genome of C. intestinalis has already been achieved (Dehal et al., 2002). Compared to the genome of humans, the ascidian genome is compact and simple (Simmen et al., 1998; Dehal et al., 2002). The haploid genome of C. intestinalis is about 160 Mbp in size and contains about 15,500 genes, just half of the human genome size and number of genes (Simmen et al., 1998). C. intestinalis is widely used as a model animal to clarify the mechanisms of embryogenesis and regulation of gene expression during the development (Satou et al., 2001; Satoh, 2003). It is of interest to clarify how DNA repair and sanitization mechanisms play a role in the prevention of the genome oxidation during development and how these systems have been conserved during evolution. It is also important to characterize the expression and regulation of the expression of BER and sanitization enzymes during the development of organisms (Vinson and Hales, 2002; Ménézo et al., 2010). As the first step to address these problems, it is necessary to identify enzymes that are involved in the repair of oxidatively damaged bases in DNA and in the sanitization of oxidized nucleotides in C. intestinalis.
We recently identified several C. intestinalis homologues of BER enzymes, CiOgg1 (Jin et al., 2006) and endonuclease III and AP endonucleases (our unpublished results). Our previous results indicated that the knockdown of CiOgg1 expression by a Morfolino oligo (RNAi) method resulted in an arrest of the early stage during development in C. intestinalis (Jin et al., 2006). In this study, we first identified a hMTH1 homologue of C. intestinalis (CiMutT). CiMutT efficiently complemented spontaneous A:T to C:G transversions in E. coli mutT mutants. Purified CiMutT showed a pyrophosphohydrolase activity that efficiently hydrolyzed 8-oxo-dGTP to 8-oxo-dGMP and pyrophosphate. However, it did not hydrolyze 2-oxo-dATP, 8-oxo-dGDP or 2-oxo-dADP. These results indicate that CiMutT is a functional homolog of E. coli MutT. The enzyme also hydrolyzed all four of the unoxidized nucleoside triphosphates (dNTPs), with a preference for dATP. The specific activity for 8-oxo-dGTP was greater than that for unoxidized dATP and dGTP. It is possible that CiMutT has the potential to protect the genome from oxidation by endogenous ROS in C. intestinalis.
Plasmid vector pGEX-4T-1 and glutathione (GSH)-Sepharose 4B were obtained from GE Healthcare Japan (Tokyo). Isopropyl-1-thio-β-D-galactopyranoside (IPTG) and KOD (Thermococcus kodakaraensis) plus DNA polymerase were purchased from Toyobo (Osaka, Japan). 8-oxo-dGTP, 8-oxo-dGDP, dGTP and dATP were purchased from TriLink BioTechnologies Inc. (San Diego, CA), Cosmo Bio (Tokyo, Japan), Takara Bio (Shiga, Japan) and Sigma–Aldrich (St. Louis, MI), respectively. Deoxyisoguanosine-5’-triphosphate (2-oxo-dATP) was obtained from ChemGenes Corp. (Wilmington, MA).
The NCBI-BLAST database was searched for proteins with amino acid sequence homology to the full length of human MTH1. Among candidates, we focused on one open reading frame (ORF), because of its high similarity to the overall amino acid sequence of hMTH1. The predicted protein, EST clone rcigd016h01, was found in the Ghost Database (Ciona intestinalis Genomic and cDNA Resources in Japan, Kyoto University). This protein was designated as CiMutT. CiMutT had an amino acid sequence characteristic of pyrophosphohydrolases for 8-oxo-dGTP. A DNA fragment (528 bp) containing the ORF for CiMutT was amplified by PCR using the C. intestinalis EST clone rcigd016h01 as a template. The PCR primers used were: for forward with a BamHI site 5’-CATGGATCCATGCATCGTTACGTTCA-3’ and for reverse with an XhoI site 5’-CGCCTCGAGAATTACTAAGACACAATTTATTT-3’. The pGEX4T-1 plasmid was used as a cloning and expression vector. Amplified DNA was digested with BamHI/XhoI and cloned into the pGEX-4T-1 BamHI/XhoI site. In the resulting plasmid pGEX-CiMutT, the gene product is fused to glutathione-S-transferase (GST) in frame. The sequence of the insert was checked to verify that no mutations had been introduced by the PCR.
A single colony of E. coli CC101 mutT mutant transfected with pGEX-CiMutT was inoculated in 20 ml of LB medium containing 100 μg/ml ampicillin and grown overnight at 37°C. Ten milliliters of the overnight culture was added to 1 liter of fresh LB medium containing 100 μg/ml ampicillin and grown at 37°C until the optical density at 600 nm reached about 0.6. The cultures were further incubated for 3 hr at 25°C in the presence of 0.1 mM IPTG. The induced cells were harvested, washed and resuspended in 20 ml of buffer A [20 mM Tris-HCl (pH 8.0), 0.1 mM EDTA, 1 mM dithiothreitol (DTT) and 10% glycerol] containing 0.1% Triton X-100. The cell suspension was sonicated and the cell lysate was centrifuged at 20,000 × g at 4°C for 30 min. The supernatant was applied to a GSH-Sepharose 4B column that had been equilibrated with phosphate-buffered saline (pH 7.2, PBS). The GST-CiMutT fusion protein was eluted from the column with PBS, followed by dialysis overnight at 4°C against PBS. The GST-CiMutT thus prepared was then cleaved by thrombin by incubation for 12 hr at 4°C. The protein digest was then applied to a GSH-Sepharose 4B column. The purified CiMutT was dialyzed against buffer A at 4°C and stored at –80°C until use. Proteins were analyzed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein concentration was determined according to Bradford (1976) with BSA as a control.
E. coli CC101 and CC101 mutT cells carrying pGEX4T-1 (vector) or pGEX-CiMutT were grown in 5 ml of LB medium containing 100 μg/ml ampicillin at 37°C for 16 hr. Appropriate dilutions of each culture were spread onto LB plates with or without 100 μg/ml rifampicin, followed by incubation at 37°C for 18 hr. Spontaneous mutation frequency was calculated by dividing the number of rifampicin-resistant mutants by that of viable cells (108).
E. coli CC101 (Cupples and Miller, 1989) and CC101 mutT cells carrying pGEX4T-1 or pGEX-CiMutT were grown in 5 ml of minimal medium containing glucose (0.2%) and 100 μg/ml ampicillin at 37°C to stationary phase. Appropriate dilutions of each culture were spread onto minimal plates with glucose (0.2%) or lactose (0.2%), followed by incubation at 37°C for 48 hr. Spontaneous mutation frequency was calculated by dividing the number of Lac+ revertants by that of viable cells (108).
The enzyme activities of CiMutT were determined in 50 μl of buffer B [20 mM glycine-NaOH (pH 9.5), 5 mM MgCl2, 40 mM NaCl, 80 μg/ml BSA, 8 mM DTT and 5% glycerol] containing various amounts of nucleotide substrates. After pre-incubation at 30°C for 2 min, the purified CiMutT was added to the reaction mixture and incubated at 30°C for 15 min. The reaction was terminated by adding an equal volume of ice-cold 5 mM EDTA. Forty microliters of the reaction mixture were applied to a high-performance liquid chromatograph (HPLC) with a TSK-gel DEAE-2SW column (2.0 mm I.D. × 25.0 cm) (Tosoh, Tokyo) in an isocratic flow by an elution buffer [75 mM sodium phosphate (pH 7.0), 1 mM EDTA and 20% acetonitrile] at a flow rate of 0.19 ml/min. The nucleotides were quantified by measuring the area of UV absorbance at 254 nm for dNTPs and 2-oxo-dATP, and 293 nm for 8-oxo-dGDP and 8-oxo-dGTP.
A search for candidates for a functional homologue of MutT in the ascidian C. intestinalis was made in the NCBI-BLAST database using the full-length amino acid sequence of hMTH1. Among candidates, we focused on one ORF, KH.C11.422.v1.C.SL1-1, because of its high degree of homology to the full-length amino acid sequence of hMTH1, including the Nudix box. The EST clone rcigd016h1 was found to contain an ORF predicted to produce a protein (CiMutT) with 175 amino acids. A certain degree of sequence homology was noted between CiMutT and mammalian MTH1 proteins. CiMutT possessed 41.7% amino acid identity with human MTH1 and higher similarity (58.3%) (Fig. 1). The degrees of amino acid sequence identity/similarity were significantly greater between CiMutT and mammalian MTH1 homologues, hMTH1 (58.3%/41.7%) and mouse MTH1 (57.7%/42.3%), than between CiMutT and E. coli MutT (27.8%/20.2%). A 23-residue module from E. coli MutT, hMTH1 and CiMutT proteins are aligned in a box of Fig. 1. All of the proteins carry a highly conserved sequence (GGX5EX7REUXEEXGU)) in nearly the same region (corresponding to amino acids 36–58 of the human protein) (Fig. 1). The homologous sequence to the Nudix motif is well conserved among MutT-related proteins from E. coli MutT to human MTH1 (Shimokawa et al., 2000; Mildvan et al., 2005; McLennan, 2006).
 View Details | Fig. 1 Amino acid sequence alignment of CiMutT and its homologues using the program CLUSTLW. The homologues are human MTH1 and E. coli MutT proteins. Conserved amino acid residues are shown in black boxes. Nudix motifs are indicated by a box. Amino acid residues thought to be essential for hydrolysis activity are indicated by the + marks. |
To investigate whether CiMutT possesses the MutT-related functions, E. coli CC101 and CC101 mutT strains were transformed with pGEX-CiMutT or pGEX-4T-1 (vector). E. coli CC101 carries a lacZ mutation on the F’ episome that can revert to the wild-type Lac+ only via A:T to C:G transversions (Cupples and Miller, 1989). In MutT-deficient strains, increased concentrations of 8-oxo-dGTP in the nucleotide pool are sufficient to enhance the spontaneous mutations. The mutT mutant gives about a 1,000-fold increase of Lac+ revertants compared with a MutT-proficient strain.
In this study, mutation frequencies were determined by rifampicin resistance and LacZ+ reversion in E. coli CC101/pGEX-4T-1, CC101/pGEX-CiMutT, CC101 mutT/pGEX-4T-1 and CC101 mutT/pGEX-CiMutT. E. coli CC101 mutT/pGEX-CiMutT showed a great decrease of the spontaneous mutation frequency (Fig. 2). The frequency of mutations to Lac+ was significantly suppressed compared with those in E. coli CC101 mutT mutant with a vector plasmid. The expression of CiMutT did not affect the types of spontaneous base substitutions other than A:T to C:G transversions in E. coli (data not shown). Therefore, it was evident that CiMutT specifically suppresses A:T to C:G transversions.
 View Details | Fig. 2 Complementation of the mutator phenotype in E. coli mutT mutant by expression of CiMutT. Mutation frequencies were determined by testing rifampicin resistance (A) and Lac+ reversion (B). E. coli CC101 and CC101 mutT cells carrying pGEX4T-1 (vector) or pGEX-CiMutT were grown in LB medium containing 100 μg/ml ampicillin at 37°C for 16 hr. Appropriate dilutions of each culture were spread : (A) onto LB plates with or without 100 μg/ml rifampicin, followed by incubation at 37°C for 18 hr, or (B) onto minimal plates with glucose (0.2%) or lactose (0.2%), followed by incubation at 37°C for 48 hr. The values represent the mean ± standard deviation (n = 4). P values were < 0.05, indicating significant difference at the 95% confidence level. |
CiMutT was overexpressed as a GST-fused protein in E. coli CC101 mutT cells. mutT mutant cells were used to avoid contamination with E. coli MutT during purification of CiMutT protein. The CiMutT-GST fusion protein was purified with GSH-Sepharose column chromatography (Fig. 3). GST-CiMutT with an apparent molecular weight of ~45 kDa was cleaved with thrombin, and CiMutT protein was then purified with a GSH-Sepharose column. The purified protein (20. 4 kDa) was used for further enzyme activity assays.
 View Details | Fig. 3 Expression and purification of the CiMutT protein. E. coli CC101 mutT cells carrying pGEX-CiMutT were induced by 0.1 mM IPTG for 12 hr. Proteins were separated by 15% SDS-PAGE and stained with Coomassie Blue. Lane 1, soluble fraction of cell extract prepared from E. coli CC101 mutT; lane 2, soluble fraction of cell extract from E. coli CC101 mutT cells with pGEX-CiMutT; lane 3, flow-through fraction from GSH-Sepharose column; lane 4, purified CiMutT after digestion of the CiMutT-GST fusion protein with thrombin; lane 5, GST-CiMutT fusion protein; lane 6, molecular weight marker proteins (175, 83, 62, 47.5, 32.5 and 25 kDa). |
To investigate the enzyme activity of CiMutT, the reaction mixture (50 μl) containing 20 mM Tris-HCl (pH 8.0), 4 mM MgCl2, 40 mM NaCl, 8 mM DTT, 80 μg/ml BSA, 5% glycerol and each substrate at 20 μM was incubated with purified CiMutT at 75 nM at 37°C for 15 min. The products were applied to an HPLC column with a TSK-gel DEAE-2SW column (2.0 mm I.D. × 25.0 cm) in an isocratic flow with elution buffer [75 mM sodium phosphate (pH 7.0), 1 mM EDTA and 20% acetonitrile] at a flow rate of 0.19 ml/min. The HPLC profiles are shown in Fig. 4A. A peak corresponding to 8-oxo-dGTP was detected at 24 min of elution time (Fig. 4A). After incubation with CiMutT at 37°C for 15 min, the peak at 24 min markedly decreased and the peak at 11 min corresponding to 8-oxo-dGMP increased. The disappearance of 8-oxo-dGTP was thus reflected in the appearance of 8-oxo-dGMP. The amount of inorganic pyrophosphate was also increased (data not shown). 8-oxo-dGDP was not detected after incubation of 8-oxo-dGTP with purified CiMutT. Therefore, we concluded that CiMutT has a pyrophosphohydrolase activity that degrades 8-oxo-dGTP to 8-oxo-dGMP and inorganic pyrophosphate.
 View Details | Fig. 4 The pyrophosphohydrolase activity of CiMutT for 8-oxodGTP and 2-oxo-dATP. (A) A typical chromatogram of 8-oxo-dGTP and its hydrolyzed product. The oxidized nucleotides (20 mM) were incubated with 0 nM (a) or 75 nM (b) purified CiMutT at 30°C for 15 min. The elution times of 8-oxo-dGTP and 8-oxo-dGMP are indicated. (B) The dependence of the nucleotide hydrolysis activity on the amount of purified CiMutT. 8-oxo-dGTP (●) and 2-oxo-dATP (■) at 20 mM were incubated with various amounts of purified CiMutT at 30°C for 15 min. |
Of the oxidized nucleotides examined, 8-oxo-dGTP was efficiently hydrolyzed by CiMutT, while 2-oxo-dATP was not degraded by this enzyme, as shown in Fig. 4B. The specific activities were 11.8 and < 0.20 nmoles products/min/ng protein for 8-oxo-dGTP and 2-oxo-dATP, respectively, under the same reaction conditions. CiMutT did not hydrolyze 8-oxo-dGDP or 2-oxo-dAMP (data not shown). The optimal pH of CiMutT pyrophosphohydrolase was determined in the reaction mixtures at various pH containing 75 nM of purified CiMutT and 20 μM 8-oxo-dGTP. The reaction mixtures were incubated at 30°C for 15 min. As shown in Fig. 5A, CiMutT had a pH optimum of 9.5 and required Mg++, with the optimal activity at 5 mM (data not shown). The distinctly alkaline pH optimum was similar to those seen in the pH versus rate profiles of other Nudix hydrolase enzymes, MutT, hMTH1 and Orf135 (Xia et al., 2005; McLennan, 2006). The optimal temperature was about 30°C (Fig. 5B).
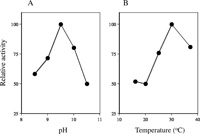 View Details | Fig. 5 The pyrophosphohydrolase activity of CiMutT for 8-oxodGTP under the conditions of various pHs and temperatures. 8-oxo-dGTP (20 mM) was incubated with 75 nM of purified CiMutT in the reaction mixture at various pHs at 30°C for 15 min (A), and incubated with 75 nM purified CiMutT for 15 min at various temperatures (B). |
To determine the kinetics of the pyrophosphohydrolase activity of CiMutT for 8-oxo-dGTP, 5–200 μM substrates were incubated with 40 nM of CiMutT, and the activity was measured for 5–60 min. The Michaelis constant (Km) and the catalytic turnover constant (kcat) were determined from the Lineweaver-Burk plots. The results are shown in Table 1. The Km value of the reaction was 16.9 μM. Therefore, the affinity of CiMutT for 8-oxo-dGTP was similar to that of human MTH1 (15.2 mM) (Fujikawa et al., 1999) and yeast YLR151 (23.8 mM) (Nunoshiba et al., 2004).
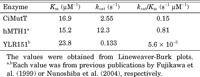 View Details | Table 1. The kinetic parameters of the pyrophosphohydrolase activity of CiMutT and for 8-oxo-dGTP |
When various unoxidized dNTPs were incubated with CiMutT, the corresponding monophosphates were generated. The amounts of the hydrolyzed products were in the order of dATP>dGTP>dCTP, dTTP (Table 2). When 8-oxo-dGTP was incubated with purified CiMutT under the same conditions as described in Table 2, 78.2% of 8-oxo-dGTP was hydrolyzed, which was more efficient than the hydrolysis of dATP (34.8%) (Table 2). Thus, it was evident that CiMutT acts as a functional homologue of MutT. The enzyme must be involved in nucleotide pool sanitization in C. intestinalis.
 View Details | Table 2. The pyrophosphohydrolase activity of CiMutT for unoxidized nucleoside triphosphates |
To prevent the deleterious effects of oxidatively damaged nucleotides, aerobic organisms have evolved the sanitization enzymes. MutT and its homologues have been identified in various organisms. The biological role of all of the MutT homologues is to remove such mutagenic nucleotides as 8-oxo-dGTP before being incorporated into DNA (Maki and Sekiguchi, 1992; Hayakawa et al., 1995; Sekiguchi and Tsuzuki, 2002; Ishibashi et al., 2003). These enzymes shows a distinct substrate specificity. Like E. coli MutT, CiMutT hydrolyzed 8-oxo-dGTP, while it was unable to degrade 8-oxo-dGDP and oxidized forms of dATP. Thus, we designated the C. intestinalis homologue of hMTH1 as CiMutT. The mammalian counterpart of MutT, MTH1, has a broader substrate specificity than MutT. hMTH1 and mouse MTH1 efficiently degrade 2-oxo-dATP and 8-oxo-dATP in addition to 8-oxo-dGTP (Maki and Sekiguchi, 1992; Furuichi et al., 1994; Sakai et al., 2002; Yoshimura et al., 2003). The broad substrate specificity of MTH1 suggests its versatile roles in multiple sanitization pathways. Furthermore, human NUDT5 specifically degrades 8-oxo-dGDP (Sekiguchi and Tsuzuki, 2002; Ishibashi et al., 2003; Kamiya et al., 2009). All of these proteins significantly suppress the mutator phenotype in mutT-deficient E. coli mutant cells (Ishibashi et al., 2003; Kamiya et al., 2009). Thus, it is quite possible that other oxidized nucleotides with potent mutagenicity are continually generated by ROS in E. coli and eukaryotes, including C. intestinalis. E. coli has three kinds of sanitization enzymes, MutT, Orf137 and Orf17, that degrade oxidized nucleotides (Maki and Sekiguchi, 1992; O’Handley et al., 1996; Kamiya et al., 2003; Xia et al., 2005; Hori et al., 2005). Hence, C. intestinalis may possess several enzymes that degrade oxidized nucleotides such as 2-oxo-dATP and 8-oxo-dGDP. Studies aiming to identify enzymes that hydrolyze these oxidized nucleotides in C. intestinalis are now underway in our laboratory.
CiMutT had activity to degrade unoxidized dNTPs with a preference for dATP over the other unoxidized dNTPs (Table 2). This unique activity has also been found in E. coli Orf17 protein (O’Handley et al., 1996). The Orf17 protein catalyzes the hydrolysis of dATP through a nucleophillic attack at the β-phosphorus to produce dAMP and inorganic pyrophosphate. This enzyme was found to be a novel nucleoside triphosphate pyrophosphohydrolase with high efficiency. However, the expression of Orf17 could not suppress the mutator activity of E. coli mutT mutant (O’Handley et al., 1996), suggesting that the nucleoside triphosphatase activity may not be involved in antimutagenesis. At present, no specific physiological function can be ascribed to the Orf17 or CiMutT protein.
MTH1 is a member of the Nudix hydrolase superfamily. Like other members, CiMutT requires Mg++ for activity and contains the Nudix motif, a highly conserved 23-amino acid residue block (GX5EX7REUXEEXGU), where U = I, L or V (Shimokawa et al., 2000; Mildvan et al., 2005; McLennan, 2006), as shown in Fig. 1. This region may function as a metal binding and catalytic site for the enzymes. Two amino acids (Trp-117 and Asp-119) in the C-terminal region of MTH1 are essential for recognizing 8-oxo-dGTP and 2-oxo-dATP (Sakai et al., 2002). Furthermore, by saturation mutagenesis of conserved residues in the MutT module, four of the 10 conserved residues (Gly37, Gly38, Glu53 and Glu57) were revealed to be essential for suppressing spontaneous A:T to C:G transversions in an E. coli mutT mutator strain (Shimokawa et al., 2000). These results suggest that CiMutT has the same substrate-binding pockets as human and mouse MTH1. The most distinctive structural difference between MutT and MTH1 is the presence of a beta-hairpin, which is absent in MutT (Sakai et al., 2002). This results in a much deeper and narrower substrate binding pocket. CiMutT has the same amino acids as human MTH1 and mouse MTH1 (Fig. 1). Structurally, this enzyme adopts a similar fold to MutT despite the low sequence similarity outside the conserved Nudix motif.
The loss of such sanitization enzymes as MutT would result in a great increase in the amount of 8-oxodG in DNA. The level of 8-oxodG in genomic DNA is significantly increased in knockout nudx-1 mutants compared with the wild-type under normal and oxidative stress (3 μM paraquat) conditions in the plant Arabidopsis thaliana (Yoshimura et al., 2007). How do cells remove a deleterious 8-oxodG incorporated into the nascent strand opposite dA during DNA replication? MutM and Ogg1 recognize and remove 8-oxodG preferentially from 8-oxodG/dC pairs, but they cannot remove 8-oxodG from 8-oxodG/dA pairs (Michaels et al., 1991; Boiteux and Radicella, 1999; Hirano, 2008). Hence, another DNA glycosylase activity must be efficient in removing 8-oxodG from 8-oxodG/dA pairs. Recently, Yonekura et al. (2007) found that endonuclease III removes 8-oxodG preferentially from 8-oxodG/dA mispairs, suggesting its involvement in removing 8-oxodG incorporated into the nascent strand opposite dA. Suzuki et al. (2008) recently showed that spontaneous mutations are greatly enhanced in E. coli mutM nth double mutants compared with the mutM and nth single mutants. These results support the notion that there is a BER pathway for 8-oxodG present in DNA as a result of incorporation of 8-oxo-dGTP opposite dA during replication. Clarifying the repair mechanism for 8-oxodG in the nascent strand will require further investigation.
A loss or decrease in MTH1 activity is considered to be deeply involved in mutagenesis and tumorigenesis, aging and other diseases (Tsuzuki et al., 2001; Sekiguchi and Tsuzuki, 2002; Yoshimura et al., 2003; Rai et al., 2009). However, little is known about how DNA damage processing influences development and growth at early stages. DNA repair mechanisms are particularly important at fertilization, immediately thereafter, and at the very onset of embryonic development (Ménézo et al., 2010). We previously observed that C. intestinalis with knock-down of CiOgg1 by the RNAi method showed arrest of development at early stages (Jin et al., 2006). To examine the role of CiMutT activity in vivo, disruption of the CiMutT gene by certain transposons and/or knockdown of the expression of the gene using the Morpholino oligo method are underway in our laboratory.
In previous studies we observed that CiOgg1 is highly expressed in the cleaving embryo stage during the development of C. intestinalis (Jin et al., 2006). Interestingly, EST analysis using the Ghost Database (Ciona intestinalis Genomic and cDNA Resources in Japan, Kyoto University) showed that CiMutT is highly expressed in the early stages during development. It will be very interesting to know how the expression of CiOgg1 and CiMutT is regulated during the development and the regulation of enzymes influence development in C. intestinalis. The higher oxygen consumption may correlate with the higher level of oxidative stress, and the level of expression of the CiOgg1 and CiMutT genes must be regulated to respond to the higher oxidative stress. Experiments leading toward this goal are in progress.
The authors thank Dr. Elizabeth Nakajima and Dr. Shuji Yonei for critically reading the manuscript and for helpful discussions. The C. intestinalis EST clone was kindly provided by Dr. Nori Satoh and Dr. Yutaka Satou, Kyoto University. Bacterial strain ASKA clones (mutT) were provided by National BioResource Project (NIG, Japan): E. coli. The authors thank Mr. Daisuke Kusano and Mr. Masahiro Kikuchi for helping with the gene cloning. This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (19510056, to Q-M. Z-A) and Global Center of Excellence Program ‘Formation of a Strategic Base for Biodiversity and Evolutionary Research (A06): from Genome to Ecosystem’.
|