| Edited by Etsuko Matsuura. Yukio Minamiya: Corresponding author. E-mail: b0mf0252000@yahoo.co.jp |
Although earthworms are hermaphroditic animals with biparental sexual reproduction, there are some parthenogenetic species, including at least two families of Lumbricidae and Megascolecidae. One advantage of parthenogenesis is the number of offspring produced (Williams, 1975; Maynard Smith, 1978). In sexual reproduction, two individuals produce one offspring per unit of time, but each parthenogenetic individual produces one offspring. Considering the distributional expansion, parthenogenesis appears to be quite advantageous, allowing for successful exploitation of environments, high colonizing ability, and rapid population growth because only one individual is necessary for reproduction (Williams, 1975). Earthworms have some reproductive organs and their terminalia openings, e.g. spermathecal pores and male pores. Spermathecal pores are the terminalia openings of spermathecae which stored sperm received from a copulatory partner until extrusion during laying. Male pores are the terminalia openings of the male organs which carry sperm towards the exterior. Since male reproductive organs are not needed for parthenogenesis, parthenogenetic earthworms show a common evolutionary trend towards a reduction in reproductive organs, such as male pores, spermathecae, and spermathecal pores (Stephenson, 1930; Gates, 1956; Jaenike and Selander, 1979). In Japan, eight earthworm species belonging to the Megascolecidae pheretimoid species lacked or had degraded male pores (Blakemore, 2003). Some studies suggested that these species are parthenogenetic (Kobayashi, 1937; Ishizuka, 2001). These earthworms show extremely low male pore possession rates (< 10%).
Amynthas vittatus (Goto and Hatai, 1898) is one of the most common earthworm species in Japan and is distributed throughout Korea and Japan, except in the Ryukyu archipelago (Blakemore, 2003). This earthworms species “originally” possessed two pairs of spermathecal pores between the 6th and 7th and the 7th and 8th segmental furrows, one pair of male pores in the 18th segment, 2–3 small genital papillae, which located adjacent male pores and spermathecal pores, and striped body markings (Hatai, 1929; Ishizuka, 2001; Blakemore, 2003). However, many studies indicated that almost all individuals of this species lacked parts of the spermathecal pores and male pores and had degraded spermathecae (Hatai, 1929; Adachi, 1955; Yamaguchi, 1962; Ishizuka, 2001). For example, Ishizuka (2001) revealed that only 20 of 311 individuals of A. vittatus collected in Tokyo possessed one pair of male pores. However, we found that almost all individuals found at Mt. Aobayama in the Miyagi prefecture in northeastern Japan possessed male pores. Gates (1956) suggested that hermaphroditic biparental reproduction is the most evolutionarily ancient form of parthenogenesis in earthworms and that not all reproductive organs were degraded. Therefore, although individuals possessing male pores are considered to be phylogenetically primitive, whether this is indeed the case remains unclear.
Recently, numerous molecular phylogenetic studies on earthworms have been conducted (Jamieson et al., 2002; Pop et al., 2003, 2007; Heethoff et al., 2004; Chang and Chen, 2005; Pérez-Losada et al., 2005, 2009; Chang et al., 2005, 2007, 2008, 2009; Admassu et al., 2006; Huang et al., 2007; King et al., 2008; Cameron et al., 2008; Minamiya et al., 2009; Richard et al., 2009; Rougerie et al., 2009; Novo et al., 2009, 2010; Knott and Haimi, 2010). Many of these studies used the mitochondrial DNA (mtDNA) cytochrome oxidase subunit I (COI) gene (Pop et al., 2003, 2007; Chang and Chen, 2005; Pérez-Losada et al., 2005, 2009; Admassu et al., 2006; Chang et al., 2007, 2008, 2009; Huang et al., 2007; King et al., 2008; Cameron et al., 2008; Minamiya et al., 2009; Richard et al., 2009; Rougerie et al., 2009; Novo et al., 2009, 2010; Knott and Haimi, 2010) because of the ease of primer design and the range of phylogenetic signals and rapid evolutionary rate of this gene (Hebert et al., 2003). To clarify the phylogenetic relationships of A. vittatus with different degree of degraded reproductive organs, we examined the morphological variations, such as the possession rate of both the male and spermathecal pores in A. vittatus found in various locations in Sendai city around Mt. Aobayama. We then constructed phylogenetic trees using mtDNA sequences from these populations.
For morphological analyses of the spermathecal and male pores of A. vittatus, 27 individuals of a single population were collected in 2007 in Sendai city, Miyagi prefecture, in northeastern Japan, and 77 individuals representing six populations were collected from the same city in 2008 (Fig. 1). After being anesthetised in 40% ethanol, the earthworms were fixed in 10% formalin and preserved in 5% formalin. Muscle tissues from three individuals in each population were isolated and frozen for DNA extraction before formalin fixation. For phylogenetic analyses, 48 individuals representing 20 populations of A. vittatus were collected from various locations in Japan (Table 1). The earthworms were anesthetised in a 40% ethanol solution. Some muscle tissue was isolated and preserved frozen for DNA extraction. The remaining samples were fixed in 10% formalin and preserved in 5% formalin.
 View Details | Fig. 1 Geographical distribution of cytochrome oxidase subunit I (COI) haplotype frequencies in A. vittatus. The size of the circles indicates the number of individuals in the population. For abbreviations, see Table 1. Shading indicates forested areas. H1 to H4 were indicated to haplotypes of Fig. 2 and Table 4. |
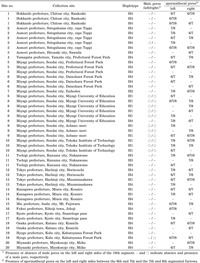 View Details | Table 1 List of source collection sites, haplotypes, and morphologies of the samples used in phylogenetic analyses |
The presence of secondary reproductive organs, such as the spermathecal and male pores, in A. vittatus was compared in each population collected in Sendai (Table 1). The external morphology of the earthworms was examined with stereoscopic microscopes (Nikon SMZ-10, Nikon Inc., Japan).
After muscle tissue samples were washed with distilled water, DNA extractions were performed using QIAGEN DNeasy™ kits (Qiagen, Valencia, USA), following the manufacturer’s protocol for animal tissue samples. The isolated DNA was resuspended in TE buffer and stored at –20°C until use. In all samples, a 690-base fragment in the protein coding region of the COI gene was amplified using the primers which were designed by Minamiya et al. (2009): Meta-2F (5’-ATRCCAGTATTYATTGGDGG-3’) and Meta-1R (5’-CTRAATACTTTRATTCCTGT-3’). Double-stranded DNA was amplified by PCR by incubating at 94°C for 10 sec, followed by 45 cycles of incubations at 94°C for 1.5 min, 48°C for 2 min, and 72°C for 3 min, with a final extension at 72°C for 15 min. PCR was performed in a 50-μL reaction volume containing approximately 50 ng total DNA, 10 mM Tris-HCl buffer (pH 8.3) with 50 mM KCl and 1.5 mM MgCl2, 0.2 mM of each dNTP, 1.25 units Taq DNA polymerase (TAKARA, Japan), and 0.5 μM of each primer. After amplification, reaction mixtures were subjected to electrophoresis on 1% low-melting temperature agarose gels and purified using QIAGEN QuickSpin™ kits following the manufacturer’s specifications. The purified PCR products were sequenced using a Big DYE-Terminator Cycle Sequencing Kit v3.1 (ABI PRISM DNA Sequencing Kit; Perkin-Elmer, Applied Biosystems, USA) and an ABI PRISM 3100-Avant Genetic Analyzer according to the manufacturer’s instructions. The same primers were used for sequencing as for amplification.
To construct phylogenetic trees, the sequences were aligned by Clustal W (Thompson et al., 1994) using default settings. The positions of deletions or insertions were manually determined. Alignment began at position 240 of Lumbricus terrestris (Linnaeus, 1758), retrieved from GenBank (accession no. U24570 (Boore and Brown, 1995)).
To assess the relationships between haplotypes, we constructed phylogenetic trees from the DNA sequences using both Neighbour-joining (NJ) and Bayesian (BI) methods. The best-fit model of sequence evolution was calculated using the hierarchical likelihood ratio tests (hLRT) with PAUP 4.0b10 (Swofford, 2002) and MrModeltest v2.3 (Nylander et al., 2004). The data set was divided into partitions corresponding to codon position (1st, 2nd, and 3rd), and model parameters were estimated separately for each position. The general time reversible model (GTR; Rodriguez et al., 1990) with a gamma-shape parameter of 0.1655 (GTR + G) was chosen (base frequencies: A, 0.2980; C, 0.2358; G, 0.1644; and T, 0.3018; substitution rates: A–C, 7.5004; A–G, 25.5331; A–T, 14.2812; C–G, 1.0942; C–T, 62.8931; G–T, 1.0000). NJ analysis was performed using PAUP 4.0b10 and the best-fit model. For NJ analyses, confidence in tree topology was tested by non-parametric bootstrap analysis (Felsenstein, 1985) with 1000 replicates. For BI analyses, posterior probabilities were obtained from a Metropolis-coupled Markov chain Monte Carlo simulation (two independent runs; four chains with 3.0 × 106 generations each; chain temperature: 0.2; trees sampled every 100 generations; deviation of split frequencies < 0.01), with parameters estimated from the data set. A consensus topology was calculated for 4500 trees after discarding the first 1500 trees (burn-in = 750) using MrBayes v3.1.2 (Huelsenbeck and Ronquist, 2001). Corrected evolutionary distances were computed using PAUP 4.0b10 and the best-fit model including all codon positions.
We analysed the male pore possession rate, defined in the present study as the occurrence of individuals with more than one male pore, in A. vittatus collected in Sendai. The male pore possession rate significantly differed among the populations from two areas, which were separated by the Hirose River (Fig. 1); almost all individuals collected at Mt. Aobayama and Mt. Yagiyama (sites 8–10) possessed male pores, but no individuals collected at sites 5–7 did (Fig. 1, Table 2). In the 2007 sample, 26 of the 27 individuals collected at Mt. Aobayama (site 8) possessed male pores, and only one lacked male pores. In the 2008 sample, 61 of the 62 individuals collected at Mt. Aobayama and Mt. Yagiyama (sites 8–10) in 2008 possessed male pores, and only one individual did not. This result is similar to the 2007 finding. In contrast, none of the 15 individuals collected at sites 5–7 (Fig. 1) possessed male pores.
 View Details | Table 2 Occurrence of male pores in A.vittatus |
Almost all individuals collected from each site around Sendai, including sites 5–7, lacked parts of the spermathecal pores. In general, some A. vittatus individuals possess two pairs of spermathecal pores (Hatai, 1929; Ishizuka, 2001; Blakemore, 2003), and we found that six individuals (6%) possessed two pairs of spermathecal pores. Moreover, 33 individuals (32%) possessed no spermathecal pores (Table 3).
 View Details | Table 3 Occurrence in spermathecal pores of A. vittatus |
COI sequences were determined from a total of 48 individuals representing 20 collection sites. We added our results to a previously published GenBank sequence (accession no. AB425818 [Minamiya et al., 2009]). The length of the sequences was 690 bp, without insertions or deletions. Of the 690 nucleotide positions, eight were polymorphic and parsimony-informative, and a total of four haplotypes were detected, named H1, H2, H3, and H4 (GenBank accession nos: AB537425–AB537427 and AB425818, respectively; Table 4). Phylogenetic trees were constructed based on both NJ and BI methods (Fig. 2). A. vittatus monophyly was fully supported by both trees (100% support in both analyses). The topologic orders of the A. vittatus haplotypes were incongruent with both trees. In the NJ tree, haplotype H4 was separated basally with relatively strong support (bootstrap value = 78%; Fig. 2), however, haplotypes H3 and H4 were basally separated from H1 and H2 in the BI tree (posterior probabilities < 61%; Fig. 2). Corrected genetic distance calculated from each haplotype was 0.00–0.13. H4 was most common haplotype, found at 16 of the 20 collection sites distributed all over the Japan (Fig. 1, Table 1). However, haplotype H1 was limited to eastern Japan (Fig. 1, Table 1). The remaining haplotypes were rare. H3 was found in only two populations, and H2 was found in a single population (Fig. 1, Table 1). Haplotype diversity was high in eastern Honshu. In Sendai, the dominant haplotype varied at each collection site. All samples from sites 5 and 6 were H4 haplotype, but almost all samples from Mt. Aobayama and Mt. Yagiyama (sites 8–10) were H1, except an H4 haplotype individual (Fig. 1, Table 1). All H1 haplotype individuals collected from Mt. Aobayama and Mt. Yagiyama possessed male pores, but the H4 haplotype individual collected from site 8 did not (Table 1).
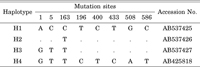 View Details | Table 4 Nucleotide sequences of unique haplotypes |
 View Details | Fig. 2 Phylogenetic tree of A. vittatus and outgroups. Solid square indicate that species (lineage) never degraded their reproductive organs, open square indicate that species (lineage) frequently degraded their reproductive organs. (A): NJ tree. The numbers below and above the branches indicate bootstrap values (> 50%). (B): Bayesian tree. The numbers below and above the branches indicate posterior probabilities (> 50%). |
Earthworms are hermaphroditic animals with biparental sexual reproduction. However, parthenogenesis with degradation of reproductive organs is not uncommon. Many studies examined the ecology and genetics of parthenogenetic earthworms, including two families of Lumbricidae and Megascolecidae (Lumbricidae: Jaenike and Selander, 1979; Terhivuo, 1988; Terhivuo and Saura, 1990, 1993a, 1993b, 2003, 2006; Mezhzherin et al., 2008; Megascolecidae: Gates, 1956). Parthenogenetic earthworms evolutionarily trend towards reduction of reproductive organs, especially male organs (e.g. Stephenson, 1930; Gates, 1956; Jaenike and Selander, 1979; Sims and Gerard, 1985). In pheretimoid species of the Megascolecidae family, individuals with degraded reproductive organs were reported from more than 10 species (Ohfuchi, 1938, 1939; Gates, 1956, 1972; Ishizuka, 2001). These studies reported that the possession rate of the male pore were extremely low (< 10%), and the possession rate of the original pairs of spermathecal pores varied in each species. In fact, Ishizuka (2001) indicated that almost all Pheretima irregularis (= A. tokioensis) and P. vittata (= A. vittatus) individuals lacked parts of spermathecal pores, but some species, including P. agrestis, P. aokii, P. hilgendorfi, and P. striata retained complete original spermathecal pore pairs. Moreover, except for A. tokioensis, we could not discover individuals with degraded reproductive organs in the earthworm species we used as outgroups (Fig. 2). Therefore, we considered that earthworms with male reproductive organs were primitive.
Although A. vittatus originally possessed one pair of male pores, only 20 of 311 individuals collected in Tokyo (central Japan) had them (Ishizuka, 2001). Moreover, other reports could not find any A. vittatus individuals possessing a pair of male pores, and found that less than 20% of the individuals had a single male pore (Hatai, 1929; Adachi, 1955; Yamaguchi, 1962; Minamiya, unpublished data; Table 5). Compared to these previous studies, the possession rate of male pores was amazingly high in individuals collected at Mt. Aobayama and Mt. Yagiyama in our study (97.8%; Table 5). This specific result was seen for two consecutive years (2007 and 2008) at site 8, indicating that this was not an accidental situation caused by environmental changes or similar factors. In contrast to male pore possession rate, few individuals collected at Mt. Aobayama and Mt. Yagiyama (sites 5–7; Fig. 1) possessed two original pairs of spermathecal pores, supporting the results of previous studies (Hatai, 1929; Adachi, 1955; Yamaguchi, 1962; Ishizuka, 2001; Minamiya, unpublished data, Table 6). Individuals from Mt. Aobayama and Mt. Yagiyama possessed the degraded spermathecal pores, and so that they could not perform biparental sexual reproduction without parthenogenesis.
 View Details | Table 5 A comparison of male pore possession rates (%) of A. vittatus |
 View Details | Table 6 A comparison of spermathecal pore possession rates (%) of A. vittatus |
Our phylogenetic analysis revealed that, except for the single individual lacking male pores, the individuals collected from Mt. Aobayama and Mt. Yagiyama are genetically distinct from those collected from other sites around Sendai, but did not indicate ancestral position of this population (Fig. 1, Fig. 2). Based on morphological and molecular phylogenetic analyses, it was considered that populations without spermathecal and male pores had not been originated from populations in Mt. Aobayama and Mt. Yagiyama, but from populations conducting biparental sexual reproduction without parthenogenesis. Therefore, the parthenogenetic earthworm A. vittatus has undergone at least two morphological evolutionary processes; only spermathecal pores were degraded or male pores were reverted after the degration of spermathecal and male pores in one lineage (H1), and both spermathecal and male pores were degraded in the other. However, unfortunately we could not discover the population, which performed biparental sexual reproduction without parthenogenesis, at the basal position of our phylogenetic tree.
The genetic distances between A. vittatus haplotypes were lower (0.00–0.13) than those of other species within the same genus (0.15–0.16 [Admassu et al., 2006]; 0.16–0.23 [Huang et al., 2007]; 0.15–0.244 [Chang et al, 2007]; 0.15–0.28 [Chang et al., 2008]; 0.16–0.22 [Novo et al., 2009]). Moreover, H1 haplotype individuals were found at sites 13 and 14 as well as sites 8–10 (Fig. 1, Table 1), but individuals collected from sites 13 and 14 did not have male pores (Table 1). These individuals were allopatrically distributed with H3 and H4 haplotypes, suggesting that hybridization among these individuals had occurred. Our phylogenetic analyses were inferred using mtDNA, which represents only the maternal pedigree, so we could not detect hybridization, introgression, and mtDNA capture. Moreover, only two of the 89 individuals collected at both Mt. Aobayama and Mt. Yagiyama lacked male pores, and one of them had a distinct haplotype (H4) from the other individuals for which molecular phylogeny was determined (Fig. 2). Therefore, additional molecular markers, particularly nuclear DNA markers, should be surveyed to identify hybridization among mitochondrial phylogenetic lineages.
Populations collected at Mt. Aobayama and Mt. Yagiyama were genetically distinct from the other populations collected at Sendai (sites 5–7), which correlated with male pore possession rates. Previous studies discussed the correlation between morphological traits and genetics in parthenogenetic earthworms. In some Lumbricidae earthworms, the morphological traits, such as body size and the number of segments, differed in each clone (Terhivuo, 1988; Terhivuo and Saura, 1993b), but in other species, morphological differences did not correlate with genotypic ones (Terhivuo and Saura, 1990, 1993a, 1993b, 2003, 2006). Presence of male pores did not correlate with genotype (Terhivuo and Saura, 1993a, 2006). In pheretimoid species, variability in reproductive organs has only been discussed in correlation with environmental factors, without molecular data (Ohfuchi, 1939; Takahashi, 1952). To our knowledge, ours is the first report to clarify the correlation between reproductive organ degradation and generic differences in the earthworm.
Although the geographic distance between sites 7 and 10 in our study was only 300 m, and the forested area was not disjunct, the possession rates of male pores were significantly different between the organisms collected at these sites (Table 3). Additionally, individuals collected at these two sites did not share the same haplotype. We could obtain only three individuals from site 7; therefore, more extensive surveys are needed at this site. These additional surveys could provide valuable opportunities for revealing reticulate evolutionary history of A. vittatus.
We express our sincere thanks to participants of the 9th earthworm summer school at the Miyagi University of Education for collection of earthworms. We also wish to thank Drs. J. Tsukamoto, F. Tamura, T. Ichie, K. Ito, K. Kawano, M. Nakamura, A. Usami, A. Hirata, and Y. Muramatsu for providing valuable assistance. This study was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to T. Fukuda).
|