| Edited by Hidenori Tachida. Yiquan Wang: Corresponding author. E-mail: wangyq@xmu.edu.cn |
Amphioxus (or lancelet) is a small sand-buried marine animal. It shares key anatomical and developmental features, such as hollow dorsal neural tube, notochord, perforated pharyngeal region, segmented muscle, post-anal tail, body plan establishing and organogenesis, with vertebrates. However, the genome of amphioxus have not undergone two-round genome duplication event that have occurred in vertebrates, and appeared to be a good surrogate for the ancestral chordate genome with respect to gene content, exon–intron gene structure and even chromosomal organization (Putnam et al., 2008). Thus, amphioxus occupies an important evolutionary position for understanding the origin and development of vertebrates (Holland et al., 2004; Yu et al., 2007).
Studies on the embryonic development of amphioxus have been carried out during the last decades to get more insights into the developmental mechanisms of vertebrate embryos. It was observed that several orthologues of vertebrate genes involved in D/V and A/P patterning, such as BMP, Nodal and Wnt, were also expressed in the amphioxus embryos, and were mostly in patterns comparable to those of their vertebrate counterparts (Schubert et al., 2001; Yu et al., 2007; Garcia-Fernandez et al., 2007). These suggest they are similarly deployed in amphioxus and vertebrates. Therefore, the molecular mechanism of amphioxus embryonic development should provide important clues to understand that of vertebrates.
During the process of amphioxus embryonic development, the neurula is an important stage when the fundamental differentiations of tissues and organs take place. At this stage, the non-neural ectoderm separates from the edges of neural plate and fuses dorsally over the forming neural tube. Then the neural plate rolls up into the tube that encloses a neural canal. Meanwhile, notochord and mesoderm are formed by folding from the dorsomedian and the dorsolateral wall of archenterons. Finally, a triploblastic neurula, consisting of definitive ectoderm, neural tube, notochord, endoderm, and mesoderm, is formed (Hirakow and Kajita, 1994).
To meet the need of the fundamental differentiations of tissue and organ, the expression of many important genes (including regulator gene, enzyme genes and structure gene) might increase remarkably. In order to isolate genes up-regulated at the early neurula stage after gastrula, we constructed an 11-hour neurula subtracted cDNA library using subtractive suppression hybridization (SSH) technology (Diatchenko et al., 1996). The early gastrulae cDNA was used as a driver to subtract house-keeping genes and basic development regulator genes. The expression of genes isolated from the subtracted cDNA library was further analyzed by real-time quantitative PCR (RTqPCR) and whole-mount in situ hybridization (WISH). Undoubtedly, this study will provide some new data for further investigation on amphioxus embryo development.
Amphioxus samples were identified as Branchiostoma belcheri according to the previous morphological descriptions (Zhang et al., 2006) and cultured in the laboratory. They spontaneously spawned in the laboratory during the breeding season, and fertilized eggs floated within seawater and developed synchronously at room temperature. We collected embryos and larvae at different developmental stages using 100-mesh nylon net as in the previous report (Zhang et al., 2007). About twenty embryos or larvae of each developmental stage were selected and immediately preserved in 500 μL Trizol reagent. If the samples were not employed to subsequent experiments shortly, they would be frozen in liquid nitrogen and stored at –80°C until RNA extraction. The remaining embryos and larvae were fixed in freshly made 4% paraformaldehyde confected with MOPS buffer (pH 7.5) at 4°C for 12 h, then were transferred through two changes of 70% ethanol, and stored in 70% ethanol at –20°C.
Embryonic polyA+ RNAs, extracted from 5-hour early gastrulae and 11-hour neurula using illustraTM QuickPrep Micro mRNA purification kit (GE Healthcare), were adopted to synthesize double-stranded cDNA using SMARTTM PCR cDNA synthesis kit according to the instruction of producer (BD Biosciences Clontech). A neurula subtracted cDNA library was constructed using PCR-SelectTM cDNA subtraction kit (BD Biosciences Clontech) with neurula cDNA as a tester and gastrula cDNA as a driver. To evaluate the efficiency of the cDNA subtraction, we performed RT-PCR to quantitate β-actin gene in subtracted and un-subtracted cDNAs. After secondary suppressive PCR, products were cloned into pMD-18T vector (Takara) for the construction of neurula subtracted cDNA library.
Positive clones were selected for sequencing from the subtracted cDNA library using PCR screening method. Sequencher 4.2 software was implemented to assemble the sequences using assembly parameters: minimum match percentage =95% and minimum overlap =50 bp. Then the assembled sequences (hereafter called contigs) were subjected to the following homology searching analysis. Firstly, homologues of these contigs were searched from NCBI (http://www.ncbi.nlm.nih.gov) and the amphioxus (B. floridae) genome database at JGI (http://genome.jgi-psf.org/) using BLASTX program with cutoff E-value at 1e-5. Then, the unknown contigs were employed to search against EST database at NCBI using the EST-BLAST and B. floridae cDNA database (http://amphioxus.icob.sinica.edu.tw/). All ESTs searched from two databases were imported into Sequencher 4.2 for assembling again, and sequences with a minimum overlap length of 100 bp and at least 95% identity were assembled into the same contig. Those reassembled contigs were used to search homologues again. For contigs whose homologues were still not searched out, we set cutoff E-value at 10 to do alignment search once more at JGI, NCBI and B. floridae cDNA database. Finally, the homologues of the residual unknown contigs were searched in genome databases of seven selected species, including four vertebrates (human Homo sapiens, mouse Mus musculus, clawed frog Xenopus laevis and zebrafish Danio rerio) and three invertebrates (ascidian Ciona intestinalis, insect Drosophila melanogaster and nematode Caenorhabditis elegans).
Considering the diversity among individuals, 20–30 embryos were combined for total RNA extraction using Trizol reagent (Tiangen). The RNAs of 5-hour gastrulae and 11-hour neurula were respectively extracted and treated with DNase I (RNase free, Takara) to eliminate genomic DNA contamination, and then cDNA were synthesized using PrimescriptTM RT reagent Kit (Takara) with oligo d(T) and random primers.
RTqPCR analysis was carried out using SYBR® Green Realtime PCR Master Mix (Toyobo) with primer concentration at 10 μM. The reaction conditions consisted of 95°C for 1 min followed by 40 cycles of 95°C for 15 s, 58–63°C (according to different genes) for 15 s, and 72°C for 1 min. Each reaction was performed three times. Amphioxus β-actin was employed as inner-control to normalize the starting quantity of RNA. Data were quantified using the 2-ΔΔCT method based on CT values for both analyzed and β-actin in order to calculate the fold increase (Livak and Schmittgen, 2001). We examined the expression levels of 20 different single contigs screened from the subtracted cDNA library including 10 identified and 10 unidentified genes. The primer sequences used here are listed in Table 1.
 View Details | Table 1 Primer sequences used for evaluating gene expression levels |
To evaluate the gene expression, we performed further investigations on AmphiFABP. Firstly, 5' RACE PCR was adopted to clone the full-length of AmphiFABP cDNA using primers SMART-F (5'-ATCAACGCAGAGTACGCGGG-3') and FABP-R (5'-CTCTTTTCTGCGTCACCAC-3') with neurula SMART cDNAs as templates. The 3' RACE PCR is not necessary since the sequence obtained from the subtracted cDNA library contains a poly (A) tail already.
AmphiFABP gene was translated into protein by DNAStar software, then FABP protein sequences of both amphioxus and vertebrates were aligned using CLUSTAL X. The sequence matrix was applied to generate neighbor-joining tree (Saitou and Nei, 1987) using MEGA4 with protein Poisson distances, and the reliability of each interior branch of the tree was assessed by bootstrapping with 1,000 replications.
The expression of AmphiFABP during the embryonic development was analyzed by RTqPCR and WISH methods. Total RNAs of 9 embryonic developmental stages were separately isolated using Trizol reagent (Tiangen). The manipulations of reverse transcription and RTqPCR were same as described above. The primer pair Fabp-F: 5'-CACCACCTTCAAGAACACC-3' and Fabp-R: 5'-CTCTTTTCTGCGTCACCAC-3' designed here could generate a 133 bp DNA segment. The sense and anti-sense RNA probes for AmphiFABP were synthesized using digoxigenin-UTP (DIG) RNA labeling in vitro transcription kit (Roche). Whole-mount in situ hybridization (WISH) for the amphioxus embryos and larvae were performed following the previous description (Holland, 2008).
We sequenced total of 204 clones obtained from the neurula subtracted cDNA library. The sizes of inserted sequences range from 141 to 877 bp with average at ~500 bp. Those sequences were further assembled into 82 contigs, and among them, 45 (~55%) showed sequence similarity to the genes from other organisms with 19 being highly similar to structure protein genes (such as ribosomal protein gene and histone gene), 17 similar to genes encoding regulatory functions, 4 to enzyme genes, and 5 to the sequences of other known protein genes or mitochondrial genes. However, the homologous search in the public databases did not reveal any significant matches for the remaining 37 contigs (~45%). Thus, we treated them as unidentified contigs provisionally (Table 2).
 View Details | Table 2 The results of homology search for contigs from the subtracted cDNA library |
To further assess the difference of gene expression between neurula and gastrulae, we did RTqPCR analysis for 20 selected contigs. The results showed that 14 genes of them were up-regulated in neurula with 9 being highly up-regulated (above 5 times increment) and 5 moderately up-regulated (less than 5 times increment) (Fig. 1, a and b). Of those highly up-regulated genes, number N5, N15 and N16 belong to regulator genes; N18 is an enzyme encoding gene; N52, N54, N57, N63 and N67 are unidentified contigs. Among the moderately up-regulated genes, number N8 and N13 are regulator genes, and N47, N48 and N51 are unidentified contigs. Among those identified genes, fatty acid-binding protein gene (FABP, N5) and skin calmodulin-related factor gene (N16) had about twenty-fold increment in neurula, insulin-like growth factor binding protein gene (N15) had more than hundredfold increase, and glyoxal oxidase precursor gene (N18) was only detected in neurula (Fig. 1a). For those unidentified contigs, N63 and N67 had about fivefold increment, N52, N54, and N57 had about twenty-fold to fifty-fold increase (Fig. 1, a and b). In addition, the detection also revealed 6 down-regulated genes at the neurula stage including four known genes and two unidentified contigs (Fig. 1c).
 View Details | Fig. 1 Validation of differential gene expression levels by RTqPCR. Relative expression of each analyzed gene in neurula and gastrula. Real-time PCR reactions were performed in triplicate for each analyzed gene and the error bars indicate the standard deviation of three data. a, b. The gene expression levels of 14 genes were up-regulated in the neurula; c. The gene expression levels of 6 genes were down-regulated in the neurula. |
Among those up-regulated genes, the expression of AmphiFABP dramatically increased (about twenty-fold) in the neurula. Several previous reports have indicated that B-FABP, homologue of AmphiFABP, is correlated with neuronal differentiation during the development of central nervous system in vertebrates (Feng et al., 1994; Kurtz et al., 1994; Liu et al., 2004). So we further cloned and sequenced the full-length cDNA of AmphiFABP that is 1,019 bp in length and codes for a protein of 136 amino acids (GenBank accession no. GQ180981). To clarify the evolutionary relationship between the FABP gene of amphioxus and the member of vertebrate FABP family, we searched out homologous sequences from the genome database of one ascidian (Ciona), four representative vertebrates and amphioxus B. floridae, and reconstructed an NJ tree based on deduced protein sequences (Fig. 2).
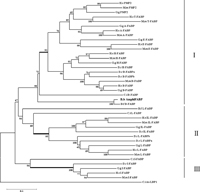 View Details | Fig. 2 Phylogenetic tree of FABP based on amino acid sequences. The tree was constructed using the neighbor-joining method. The ATP synthase lipid binding protein 1 of Ciona intestinalis was used as an outgroup. The numbers on the branches represent the bootstrap values of the clades. I, II, III show 3 groups consisting of 9 FABP proteins in vertebrates. The three bold branches show the bases of the three groups of the vertebrate FABP genes. Species codes are: H.s, Homo sapiens; M.m, Mus musculus; G.g, Gallus gallus; D.r, Danio rerio; B.f, B. floridae; B.b, B. belcheri; C.i, Ciona intestinalis. GenBank accession numbers for FABP proteins are: H.s Brain-FABP (B-FABP), NP_001437; M.m B-FABP, EDL05123; G.g B-FABP, NP_990639; D.r B-FABPa, NP_571680; D.r B-FABPb, NP_999972; C.i B-FABP, XP_002127826; B.f B-FABP, XP_002589099; H.s Heart-FABP (H-FABP), NP_694493; M.m H-FABP, NP_034304; G.g H-FABP, NP_001026060; D.r H-FABP, NP_694493; H.s Epidermal-FABP (E-FABP), NP_001435; M.m E-FABP, NP_074364; G.g E-FABP, NP_001006346; H.s Adipocyte-FABP (A-FABP), NP_001433; M.m A-FABP, NP_077717; G.g A-FABP, NP_989621; H.s Testis-FABP (T-FABP), NP_001073995; M.m T-FABP, NP_035728; H.s Peripheral myelin protein 2 (PMP2), NP_002668; M.m PMP2, NP_001025476; G.g PMP2, XP_418309; H.s Ileal-FABP (IL-FABP), AAH22489; M.m IL-FABP, NP_032401; G.g IL-FABP, XP_414486; D.r IL-FABP, NP_001002076; H.s Liver-FABP (L-FABP), AAH2228; M.m L-FABP, NP_059095; G.g L-FABP, NP_989523; D.r L-FABPa, NP_001038177; D.r L-FABPb, NP_001019822; C.i L-FABP, XP_002131187; B.f L-FABP, AAT38124; H.s Intestinal-FABP (I-FABP), NP_000125; M.m I-FABP, NP_032006; G.g I-FABP, NP_001007924; D.r I-FABP, NP_571506; C.i I-FABP, XP_002127813; C.i As-LBP1, XP_002122403. |
The phylogenetic analysis divided the members of vertebrate FABP genes into three groups. The amphioxus genes AmphiFABP and B.f B-FABP are orthologues of PMP2, T-FABP, A-FABP, E-FABP, H-FABP and B-FABP genes of vertebrates, constituting the first group, and the gene B.f L-FABP is an orthologue of vertebrate IL-FABP and L-FABP genes forming the second group. The third group contains I-FABP genes of ascidian and vertebrates only because we could not find homologous sequence of amphioxus from the present database.
Furthermore, we performed RTqPCR to detect AmphiFABP gene expression in different embryonic stages. As shown in the Fig. 3, the expression of AmphiFABP initiated at the early gastrula and became more and more conspicuous as the embryonic development proceeded. At the early neurula stage, it reached the highest level, and went down at the late neurula. This moderate expression was kept during the subsequent development.
 View Details | Fig. 3 RTqPCR analysis of AmphiFABP transcription during the amphioxus embryonic and larval development. AmphiFABP transcripts were detected from the early gastrula to larval stages. β-actin was adopted as an inner-control. Real-time PCR reactions were performed in triplicate for each sample, and the error bars indicate the standard deviation of three data. |
WISH experiments did not detect the transcripts of AmphiFABP at the 8 cell or blastula stages and even at the early gastrula (Fig. 4a). A conspicuous expression appeared in the hypoblast at the very late gastrula (Fig. 4, b and c). At the early neurula, when the neural plate became flat, transcripts appeared in the neural ectoderm at the neural plate and somite mesoblast in addition to the expression in the hypoblast like that at the late gastrula (Fig. 4, e–g). Later on, when the lateral edges of the neural plate were rolling up dorsally, the expression also appeared at the notochord, somite mesoblast and hypoblast (Fig. 4, i and k). More later on, after the non-neural ectoderm fuses dorsally over the forming neural tube, the expression was mainly focused on the somite (Fig. 4l). By the late neurula stage, the expression continued at the somite and posterior hypoblast (Fig. 4, m–o). In the 3-gill-slit larva, the transcripts were detected in the neural tube, notochord, mid-gut and hind-gut (Fig. 4q).
 View Details | Fig. 4 WISH analyses on the expression of AmphiFABP gene at the different developmental stages of amphioxus embryos. (a) Concave views of the early gastrula. (b) Dorsal view of the late gastrula. (c) Anterior view of the late gastrula. (d) Sense control of the late gastrula, which was processed and hybridized similarly in the presence of sense, instead of anti-sense probe. (e) Side and (f) anterior views of the early neurula with expression on the neural plate (arrow). (g) Dorsal views of the early neurula. (h) Sense control of the early neurula. Dorsal (i) and side (j) views of the early neurula. (k) Cross section through the level k in j, arrow indicates the somite, arrowhead indicates the notochord, and the bold arrow indicates the hypoblast. (l) Side view of the early neurula with somite in focus (arrow). (m) Side view of the late neurula. (n) Cross section through level n in m, arrow shows the expression in the somite. (o) Cross section through the level o in m, arrow shows the expression in the somite and bold arrow shows the expression in the hindgut. (p) Sense control of the late neurula. (q) Side view of 3-gill-slit larva showing the expression in the neural tube (arrow), notochord (double arrow), mid-gut (arrowhead) and hindgut (bold arrow). (r) Sense control of the 3-gill-slit larva. Scale bar = 20 μm. |
The neurula is a critical embryonic development stage of amphioxus. The fundamental body plan of animals is determined at this stage because of neural tube formation and somite emergence (Hirakow and Kajita, 1994; Benito-Gutiérrez, 2006). In this study, we constructed a forward subtracted cDNA library to investigate the genes up-regulated in the early neurula. Further sequencing and assembling revealed 82 single contigs representing the genes differentially expressed in amphioxus embryos. In order to identify these genes, we searched the public databases, including B. floridae genome database, for the homologues of each contig. Surprisingly, only 55% of those expressed genes (45 single contigs) matched the known genes but 45% (37 single contigs) did not have any counterparts even when we search them in a wide range of parameters. Such a high ratio of unidentified single contigs might be accounted by two explanations: First, during the process of the library construction, cDNA templates were subjected to the restriction enzyme Rsa I digestion, and then, we cloned and sequenced each segment. The procedure led some clones representing only 5'-/3'-UTR of mRNAs where higher divergence usually exists even between two species of amphioxus B. belcheri and B. floridae. Indeed, among those unidentified single contigs, the average length of 17 contigs composed by single clone is only 475 bp. They might be re-identified as known genes if their full-length cDNA or coding regions are sequenced. Secondly, some of those unidentified contigs might be representatives of new amphioxus genes or genes that specifically appeared in cephalochordates, which seems plausible if about 500 Myr independent evolution of this lineage is taken into account (Shu et al., 1996). In fact, we did find that contigs N57, N62 and N81 are similar to the sequences of some presumed genes in the genome database of B. floridae, but did not find their homologues in any other invertebrates or vertebrates. The results imply that current unidentified contigs might be new amphioxus genes.
Although only 45 single contigs were identified from all assembled contigs in the present study, we could characterized them as growth-metabolism group, such as enzymes and ribosomal proteins encoding genes, and development control group, such as regulator encoding genes. The second group genes would be of interest in further researches since the revelation of their function will help us to understand the construction of body plan in amphioxus. For instance, IF-D1 of amphioxus (corresponding to N2) was expressed strongly in the epidermis and neural tube in adult, and was also detectable in the neurula (Karabinos et al., 2001). The expression of rab GDI gene (corresponding to N13) was detected at the neural plate of amphioxus embryos until the end of neurulation (Sedlacek et al., 1999). The expression patterns of those genes in amphioxus suggested that their primary roles might be in early nervous system development. However, there are still lots of identified or unidentified genes remaining to be further investigated.
In the present study, we obtained the full length of an amphioxus FABP gene, AmphiFABP. Its deduced protein sequence was similar to vertebrate B-FABP (48% identity) and H-FABP (47% identity) which were attributed to the family of fatty acid-binding proteins (FABPs). Therefore, we extracted all FABP members from vertebrates, ascidians and amphioxus, and performed phylogenetic analysis using ATP synthase lipid binding protein 1 (As-LBP1) from ascidians as an outgroup. The phylogenetic tree divides the family members into three major groups (Fig. 2), which is coinciding with the classification of previous reports (Schaap et al., 2002). Moreover, the tree also indicates that the three groups diverged before the appearance of early chordates because the orthologous genes from each group were found in the ancestral chordates, amphioxus and/or ascidians (Fig. 2). In group II, vertebrate genes (IL-FABP and L-FABP) evolved from a single ancestral gene, probably via whole genome duplications that occurred in the early evolution of vertebrate lineage (Putnam et al., 2008). Genes in group I also originated from a common ancestor, but experienced two duplications, the first duplication occurred in the early evolution of vertebrate lineage (for example, B-FABP and H-FABP are the result of this event), and the second one probably happened on the origin of amniotes which produced PMP2, A-FABP and E-FABP. In group III, no duplicated gene appeared in vertebrate lineage. Moreover, we also failed to amplify or search out orthologue of group III from amphioxus in this investigation. Two explanations might explain this result: first, amphioxus dose lose the orthologue gene of group III; secondly, the current genome database of B. floridae does not supply sufficient sequence data to search the orthologue.
In vertebrates, B-FABP is abundant in the developing brain, and its distribution is spatially and temporally correlated with neuronal differentiation during the development of central nervous system (Feng et al., 1994; Kurtz et al., 1994; Liu et al., 2004). In amphioxus, the expression of AmphiFABP starts at the gastrular stage and soon reaches a high level in the early neurula when the embryonic neural plate emerges. Although the positive signal of in situ hybridization in the early neurula seems to be less strong than that in late neurula, the highest expression level detected by RTqPCR in the early neurula instead of late neurula still did not conflict with the results of in situ hybridization if the diffused expression throughout almost the entire embryo (including the neural plate, mesoblast and hypoblast) was taken into account. In situ hybridization revealed that AmphiFABP was expressed at the neural plate and tube, suggesting it might also be related to the early development of the amphioxus central nervous system. Moreover, the expression of AmphiFABP was also detected in the embryonic somite, mid-gut and hind-gut of larvae, which is similar to the expressions of vertebrate H-FABP and I-FABP (Haunerland and Spener, 2004).
This work is supported by National Natural Science Foundation of China (Grant No. 30830023) and the National High-tech Research and Development Program ("863"Program) of China (Grant No. 2008AA092602).
|