| Edited by Kyoichi Sawamura. Masa-Toshi Yamamoto: Corresponding author. E-mail: yamamot@kit.jp |
In sexually reproducing organisms, it is important to use eggs and sperm effectively so as to produce as many progeny as possible. Males generally transfer numerous sperm to females during a single copulation. In several animal species, females store the sperm received by copulation for weeks, months, or even years before using them for fertilization (reviewed by Neubaum and Wolfner, 1999b). Therefore, sperm storage—including transfer of sperm to the female sperm storage locations, maintenance of sperm functionality and release of sperm for fertilization—is vital for reproductive success in these species.
In Drosophila melanogaster, females store the sperm received by copulation and use them to fertilize eggs over the subsequent two weeks (Lefevre and Jonsson, 1962). The females have two types of organs specialized for sperm storage, a long coiled tubule called the seminal (ventral) receptacle and a pair of mushroom-shaped organs called the spermathecae (Miller, 1950). It has been reported that mated females receive thousands of sperm through a single copulation and store 500–1,000 sperm in their sperm storage organs (Fowler, 1973; Gilbert, 1981; Tram and Wolfner, 1999). Females release sperm from storage organs to fertilize ovulated eggs as the eggs enter the uterus. The stored sperm are utilized highly efficiently: only one or a few sperm are used to fertilize an egg (Lefevre and Jonsson, 1962; Peacock and Erickson, 1965; Zimmering and Fowler, 1968).
In addition to sperm, mated females receive seminal fluid components that are synthesized in the male accessory gland and ejaculatory duct. Some of these components were found to have important roles in sperm storage or the utilization of stored sperm. Among the accessory gland proteins (Acps), Acp36DE has been studied extensively and suggested to have a critical role in sperm storage as a guidance factor helping sperm move into the storage organs (Bertram et al., 1996; Neubaum and Wolfner, 1999a; Bloch Qazi and Wolfner, 2003). Recently, an additional five Acps were shown to be involved in the retention and/or release of stored sperm (Ravi Ram and Wolfner, 2007; Wong et al., 2008). Esterase 6 (EST-6), which is produced primarily in the ejaculatory duct and transferred to females by copulation (Richmond and Senior, 1981), influences the release rate of stored sperm (Gilbert, 1981). It is also possible that genes encoding sperm proteins may play these roles, although few such genes have been identified. To date, only one such gene, a Drosophila homolog of Polycystein-2 (PKD2), has been shown to be important for sperm entry into the female sperm storage organs (Gao et al., 2003; Watnick et al., 2003). In order to investigate the mechanisms of sperm storage and utilization in mated females, we isolated recessive mutations that allow males to produce normal amounts of sperm, yet cause male sterility by preventing sperm from fertilizing eggs after being transferred to females upon copulation.
Here, we describe a novel male sterile mutation of D. melanogaster, wasted (wst). wst males produce large quantities of motile sperm, which are transferred to females during copulation. The mutant sperm are transferred normally to the female sperm storage organs, but they were lost from seminal receptacles within 24 hr after copulation. Sperm stored in spermathecae were retained as usual. We further found that wst sperm stored in seminal receptacles were irregularly released and often used far more excessively at ovulation. wst sperm could enter the egg efficiently during the first 5 hr after copulation, but the entry rate decreased drastically a day after copulation, suggesting that the wst sperm lose the ability to enter eggs in the course of storage. In addition, the majority of wst sperm that entered eggs failed to undergo the nuclear decondensation essential for normal fertilization. Thus, the wst+ gene is required for the processes of stored sperm utilization, including regular sperm release from the female sperm storage organs, maintenance of fertility capability, and formation of the male pronucleus in the egg.
All cultures were raised at room temperature (22 to 24°C) on standard medium unless otherwise stated. The wst mutation was isolated from isofemale lines established from a collection on Katsunuma, Japan in 1997 (Hirai et al., 2004). Oregon-R was used as the wild-type strain. A stock bearing the don juan-GFP (dj-GFP) transgene on the third chromosome, which produces a GFP-tagged sperm tail protein (Santel et al., 1997), was kindly provided by A. Santel. A second chromosome multiple marker strain, al b dp pr c px sp/ CyO, Cy and twelve overlapping deficiencies were used to map the mutation. These deficiencies were as follows: Df(2R)arr-γ2 (49F;50C6), Df(2R)cnn (50A2;50B2) and Df(2R)8-104 (50A;50B) were kindly provided by S. Dinardo, T. Kaufman and E. D. Schejter, respectively. Df(2R)Su(z)2-1.a2 (49B8;50B2), Df(2R)Su(z)2-1.a3 (49E1; 50C4), Df(2R)Su(z)2-1.a4 (49D1;50C10), Df(2R)Su(z)2-1.b10 (49C1;50C10) and Df(2R)Su(z)3-1.j32 (49B8;50B2) were kindly provided by C.-T. Wu. Df(2R)vg135 (49A; 49F1), Df(2R)vg-B (49D3;50A2), Df(2R)CX-1 (49C1;50D1) and Df(2R)Exel7124 (49F10;50A1), and all other stocks used in this study were generously provided by the Bloomington Stock Center, Indiana University and the Drosophila Genetic Resource Center, Kyoto Institute of Technology. Genes and chromosome rearrangements are described in Lindsley and Zimm (1992) or FlyBase (Tweedie et al., 2009; http://flybase.org).
The wst mutation was first mapped by recombination with respect to al b dp pr c px sp. Females heterozygous for a chromosome carrying wst and a multiple marker chromosome were mated to wst/SM1, Cy males, and the Cy+ sons then individually mated to two females carrying a multiple marker chromosome to check their fertility and, if they were fertile, the marker composition of recombinant chromosomes.
Males of each genotype and Oregon-R females were collected within 6 hr after eclosion, stored separately in small groups (less than 30 flies) on food vials for 4–6 days until experiments started. Thirty males of different genotypes and 30 Oregon-R females were put into a fresh vial and copulated pairs were transferred to new vials. Males were discarded from vials immediately after the end of copulation to avoid multiple copulations.
Copulated females were individually put into vials and transferred to new vials every day for 10 days. To measure fertility, we counted total progeny from single females during a 10-day period after a single copulation. Eggs laid by individual females were counted immediately after transfer of females and were allowed to develop for at least 48 hr before unhatched eggs were counted to determine hatch rates. Three separate trials were performed and virgin females were used as a control for each trial.
We took advantage of the dj-GFP transgene to estimate the amount of sperm stored in female sperm storage organs by measuring the intensity of GFP fluorescence. The genital tracts of mated females were dissected in 0.7% NaCl at 1, 5, 24, 48, 120 and 240 hr after the end of copulation and were observed using epifluorescence optics on a NIKON ECLIPSE E600 microscope with a FITC filter (NIKON). Images of the sperm storage organs of each female were taken (1280 × 1024 pixels) with Image Analysis Software version 1.6.1 (HAMAMATSU) using a CCD Digital Camera system (HAMAMATSU C4742-95) at a constant condition. The images were resized to 567 × 454 pixels (20 × 16 cm) using Adobe Photoshop version 4.0J, which allowed the images to be analyzed by NIH image software. Each pixel of the images contains a value ranging from zero (bright) to 255 (dark). We used the inverted value in this paper; that is, the brightest pixel contains the value 255. The mean value (Vs) and the number of pixels (Np) in the area of the sperm storage organs were measured by NIH image software version 1.62. To correct the disparity in brightness among images, the mean values outside the sperm storage organs (Vo) were also measured. The intensity of GFP fluorescence (IGF) in the area of sperm storage organs was calculated as [(Vs-Vo) × Np].
Next, we directly counted the number of sperm stored in the sperm storage organs of mated females using a staining method essentially the same as that described in Fowler (1973). The genital tracts of mated females were dissected in 0.7% NaCl at 1, 5, 24 and 120 hr after the end of copulation, fixed and stained with 2% aceto-lacto orcein. The numbers of sperm stored in seminal receptacles and spermathecae were counted separately.
The genital tracts of mated females were dissected in 0.7% NaCl at 5 hr and 24 hr after the end of copulation and fixed immediately in methanol. The genital tracts were washed in 1x PBS (1.9 mM NaH2PO4, 8.4 mM Na2HPO4, 175 mM NaCl, pH 7.4) containing 0.05% Tween-20 (PBST), and stained with 1 μg/ml 4’, 6-diamidine-2-phenylindole (DAPI) in PBST for 5 min. After washing in PBST, the genital tracts were mounted in PBS under the coverslips. The numbers of sperm released upon ovulation were counted using epifluorescence optics on a NIKON ECLIPSE E600. Five separate trials were performed independently for both periods.
Thirty mated females were placed in the egg collection vial with petri dishes containing fresh egg-laying media (25% apple juice, 1.25% sucrose and 1.75% agar) with yeast paste. Eggs laid during 1 to 5 hr and 22 to 24 hr after copulation were collected and fixed as described in Ohsako et al. (2003). The fixed eggs were doubly stained with DROP 1.1, which recognizes the sperm in fertilized eggs (Karr, 1991; Graner et al., 1994) and with DAPI. The staining methods were essentially the same as described in Ohsako et al. (2003) with the exception that purified DROP 1.1, which was kindly provided by T. Karr, was used for the primary antibody at a 1:500 dilution and Alexa flour 488 goat anti-mouse IgG (Molecular Probes) was used for secondary antibody at a 1:200 dilution. Eggs were observed using epifluorescence optics on a NIKON ECLIPSE E600. Six separate examinations were performed.
The wasted (wst) mutation, formerly named ms(2)kn108, was isolated as a male sterile mutation from isofemale lines of a natural population (Hirai et al., 2004). Viability of both sexes and female fertility are normal. Mutant males produce as many motile sperm as Oregon-R wild type males, and no morphological abnormalities are observed in the mutant sperm at the light microscope level (data not shown). However, the wst males showed fertility that was only 1% that of control males heterozygous for wst (wst/SM1) (Table 1). The total number of eggs laid during the 10 days after copulation was 30% lower than the control and the egg hatch rate was only 1.2%. Males hemizygous for wst (wst/Df(2R)arr-γ2) showed a further reduction in fertility. The wst gene was mapped genetically to 2-71.6 between pr (2-54.5, 38B3) and c (2-75.5, 52D3-7). Complementation tests using 12 deficiencies (see MATERIALS AND METHODS) showed that the four deficiencies Df(2R)vg135, Df(2R)vg-B, Df(2R)8-104 and Df(2R)Exel7124 complemented wst, but the other eight deficiencies failed. These results indicate that the wst gene maps to the cytological interval 50A3 to 50B2.
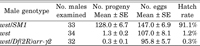 View Details | Table 1 Fertility of males bearing the wst mutation |
Copulation duration of the wst and wst/SM1 males was not significantly different (19.63 ± 0.60 min, n = 24 and 18.25 ± 0.56 min, n = 24, respectively; Student’s t = 1.675, p = 0.101). The amounts of sperm in uteri of the females mated to either wst or control males appeared to be equal immediately after copulation (data not shown), indicating that wst males transfer sperm to females as effectively as normal males.
To examine sperm storage efficiency, sperm were visualized using the dj-GFP transgene (Santel et al., 1997). As shown in Fig. 1, GFP fluorescence was observed in the sperm storage organs, seminal receptacle (SR) and spermathecae (Sp) of mated females. We observed that the fluorescence intensity of the sperm tails did not diminish for 16 days after copulation. We measured the intensity of GFP fluorescence (IGF) to estimate amounts of sperm. The IGF was expected to be proportional to the number of sperm since the fluorescence intensity of a single sperm was high enough to capture with the highly sensitive CCD camera. Shortly after copulation, females mated to wst or wst/SM1 males showed almost the same fluorescence intensity in SR and Sp (Fig. 2). However, the number of wst sperm showed a sharp decrease in SR within a few days after copulation, while sperm stored in Sp decreased slowly (Fig. 2). These results clearly indicate that wst sperm can enter the female sperm storage organs, but fail to be maintained as long as control sperm.
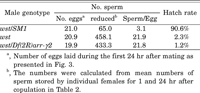
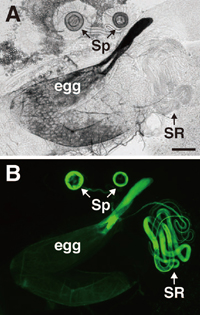 View Details | Fig. 1 Visualization of sperm stored in the sperm storage organs of a female mated to a male bearing the dj-GFP transgene. The genital tracts of mated females were dissected and observed using phase-contrast (A) or epifluorescence (B) microscopy. Sperm were stored in the specialized organs termed spermathecae (Sp) and seminal receptacle (SR). Note that the chorion, especially in the chorionic appendage of ovulated eggs, showed auto-fluorescence with the FITC filter. Bar represents 100 μm. |
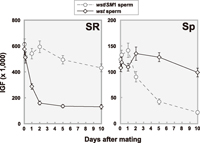 View Details | Fig. 2 Reduction patterns of stored sperm in seminal receptacles (SR) and spermathecae (Sp) over time after a single copulation. The amounts of stored sperm were estimated by intensity of GFP fluorescence (IGF) as described in MATERIALS AND METHODS. Oregon-R females were mated to wst/SM1 (open circles with dashed line) or wst (open diamonds with solid line) males bearing the dj-GFP transgene. Each point represents the average value (with standard error) of 20 to 36 mated females. |
For precise measurements, we counted directly the number of sperm in SR and Sp of females mated to wst, wst/SM1, or wst/Df(2R)arr-γ2 males without the dj-GFP transgene (Table 2). As expected, the IGF was highly correlated with the number of stored sperm (r2 = 0.921, t = 9.65, P < 0.01 for SR; r2 = 0.730, t = 4.65, P < 0.01 for Sp; see Fig. 2 and Table 2), indicating that the IGF is useful for evaluating the number of sperm stored in the sperm storage organs. In addition, it is obvious that expression of the Don Juan-GFP fusion protein does not affect overall patterns of sperm utilization.
 View Details | Table 2 Mean number of sperm stored in the sperm storage organs |
In SR, the number of sperm at 1 hr after copulation is the same among the males examined. Considering the reports that the SR reaches maximum fullness within 1 hr after the end of copulation (reviewed in Fowler, 1973 and references therein), we conclude that wst sperm can be stored normally after copulation. At 5 hr after copulation, however, about 200 sperm from wst homozygotes and hemizygotes had disappeared (Table 2). By this time, most of the mated females held an egg in their uteri, and some females did not retain an egg nor a mass of sperm in their uteri, indicating that they had already ovulated at least once. It should be pointed out that 92% of wst sperm were lost within 24 hr and by 120 hr almost no sperm were left. In contrast, only about 160 control sperm were used within 120 hr and two-thirds of the stored sperm were still retained.
In Sp, no significant decrease in the number of stored sperm was observed in all cases examined until 24 hr after copulation. By 120 hr, Sp of females mated to control and hemizygous males were reduced to a half to one-third maximum fullness, whereas Sp of females mated to wst homozygotes had released fewer sperm. The wst mutation is a loss-of-function mutation, because the sperm of the wst/Df(2R)arr-γ2 hemizygotes and wst homozygotes, except for 120 hr in Sp, had similar storage phenotypes.
In D. melanogaster, copulation stimulates egg-laying that is induced by both sperm and seminal fluid components (Manning, 1962, 1967; Kalb et al., 1993; Heifetz et al., 2001). We investigated rates of egg laying and hatching every 24 hr for 10 days after copulation. As shown in Fig. 3A, the females mated to control males laid the largest number of eggs on the first day, and egg lay gradually decreased over 10 days. Egg counts corresponded well to the numbers of progeny (Fig. 3C), since the egg hatch rate was consistently high (Fig. 3B). It was very striking that females mated to wst males laid the same numbers of eggs as the control on the first day, but reduced the number of eggs laid to the level of virgin females by the third day (Fig. 3A).
 View Details | Fig. 3 Number of eggs laid, their hatch rates and number of progeny of mated or virgin females over time. Oregon-R females were tested as virgins (open triangles with solid line, n = 30) or after mating to wst/SM1 males (open circles with dashed line, n = 33), wst males (open diamonds with solid line, n = 34), or wst/Df(2R)arr-γ2 males (closed diamonds with dashed line, n = 32). |
The hatching rate of eggs laid by females mated to wst males was extremely low (Fig. 3B). No eggs hatched on the sixth to the ninth days after copulation, but four out of 36 females produced a single offspring on the tenth day. Since virtually no sperm were left in the SR (Fig. 2 and Table 2), the offspring might be produced from sperm stored in Sp. It is interesting to note here that wst sperm are stored in the Sp even after the time when nearly all the sperm have disappeared from the SR. Sperm from wst/Df(2R)arr-γ2 hemizygous males showed very similar effects (Fig. 3). Since most wst sperm were released from the SR but most eggs were unhatched nonetheless, sperm release seemed to occur independently from ovulation.
In order to analyze the sudden decrease in wst sperm in SR, we examined the release rate of wst sperm stored in SR using a strain bearing a dj-GFP transgene. Mated females were dissected 5 or 24 hr after the end of copulation to see if an ovulated egg and a mass of sperm released from the female sperm storage organs were present together in the uterus. The results indicate that stored wst and control sperm are both released in 90% of the ovulation events (Table 3).
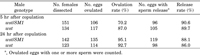 View Details | Table 3 Ovulation and sperm release |
We then asked why the amount of wst sperm in SR decreased so drastically. On average, 458 wst sperm were lost within 24 hr, while only 65 control sperm were released (Table 4). Because almost the same numbers of eggs were laid in the 24 hr after copulation, the average numbers of sperm used per ovulation were calculated as 3.1 and 22 for the control and the wst, respectively. It should be noted here that the hatch rate was extremely low in spite of the large number of the wst sperm used for each egg (Table 4).
 View Details | Table 4 Estimated sperm number per single ovulation and egg hatch rate |
The sperm released from the female storage organs were observed around the micropyle (arrowheads in Fig. 4, C to F) positioned at the boundary between the oviduct and anterior uterus where the exit of the seminal receptacle is located. The large mass of sperm tails visualized by Dj-GFP makes it obvious that more wst sperm were released than control sperm (Fig. 4, C and D).
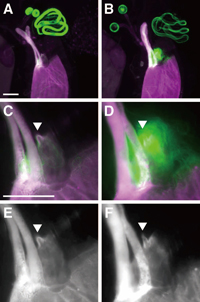 View Details | Fig. 4 Visualization of ovulated eggs and sperm released from sperm storage organs of females mated to wst/SM1 (A, C and E) or wst (B, D and F) males bearing dj-GFP transgene. Since the chorions of ovulated eggs show auto-fluorescence with both the FITC (pseudo-colored with green, see also Fig. 1) and DAPI (pseudo-colored with magenta) filters but sperm were visualized only with the FITC filter, we can easily distinguish released sperm (green) from chorion (magenta) in merged views (A to D). C, E and D, F were higher magnification views of A and B, respectively, and E, F show visualizations with only the DAPI filter. Arrowheads in C to F indicate the micropyle. Bars represent 100 μm. |
We further analyzed sperm release by counting sperm heads stained with DAPI. In the control, as summarized in Fig. 5, the variations of sperm numbers at 5 hr after copulation were equivalent to those at 24 hr (Mann-Whitney U-test, t = 0.815, 0.4 < P < 0.5). The medians were 3.5 at 5 hr and 3 at 24 hr, which agree well with the estimate described above (3.1 sperm/oviposition, Table 4), indicating that sperm release is well controlled.
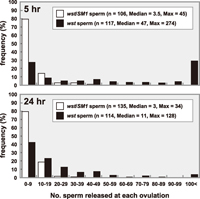 View Details | Fig. 5 The number of released sperm per ovulated egg. Sperm heads released from sperm storage organs of females mated to wst/SM1 (open bars) or wst (closed bars) males bearing the dj-GFP transgene were stained with DAPI and counted 5 hr (upper) or 24 hr (lower) after copulation. |
On the other hand, the release of wst sperm appeared to be out of control (Fig. 5). At 5 hr after copulation, the median was 47, which was 13 times as many as that of the control, and the maximum at a single ovulation was 247, corresponding to one third of the average number of stored sperm (see Table 2). At 24 hr, three times as many wst sperm were released as the control even though the number of wst sperm had decreased significantly from the number present at 5 hr (Mann-Whitney U-test, t = 5.16, P < 0.01) due to the severe depletion of sperm stored in SR (see Table 2). These results indicated that the wst sperm stored in SR were lost quickly because a large number of sperm were released at each ovulation. Nevertheless, hatching rates of eggs laid by females mated to the wst males were extremely low even during the 24 hr after copulation (Table 4), suggesting that the wst sperm could not enter into eggs or, after entering eggs, no fertilization or embryogenesis occurred.
To examine the above possibilities, we investigated sperm entry into eggs during 1 to 5 hr and 22 to 24 hr after copulation using DROP 1.1, an antibody that recognizes sperm in fertilized eggs (Karr, 1991; Graner et al., 1994). We also examined the behavior of the sperm nucleus during the fertilization process by staining with DAPI. Ninety percent of the eggs of females mated to control males were inseminated as expected (Table 5). Insemination rates correspond well to sperm release rates at 5 hr as well as 24 hr after copulation (see Table 3 and Table 5), implying that a release of sperm is enough to inseminate an egg. On the other hand, about 85% of eggs laid by females mated to wst males were inseminated during 1 to 5 hr after copulation. Although this rate was significantly lower than that of the control (χ2 = 7.41, P < 0.01), it seemed sufficiently high to conclude that an egg would be inseminated if one wst sperm was released (χ2 = 1.95, 0.5 > P > 0.1 for eggs with released sperm at 5 hr (Table 3) and inseminated eggs during 1 to 5 hr). In contrast, during 22 to 24 hr, the sperm entry rate decreased considerably despite a high rate of sperm release (Table 3) (χ2 = 52.2, P < 0.01 for eggs with released sperm at 24 hr (Table 3) and inseminated eggs during 22 to 24 hr). This suggests that wst sperm lose the ability to enter eggs during storage. Nevertheless, the rate of entry of wst sperm was too high to explain the low hatch rate, suggesting that wst sperm were defective in processes occurring after sperm entry.
 View Details | Table 5 Sperm entry rates and fertilization rates |
About 98% of eggs inseminated by control sperm began embryonic development as expected for the completion of normal fertilization (Table 5). In contrast, nuclear divisions were initiated in an extremely low proportion of eggs inseminated by sperm from homozygous or hemizygous wst males. This indicates that the low hatch rate was mostly due to a failure of fertilization.
In the eggs that did not initiate mitotic divisions, DAPI-staining showed the presence of the three polar body nuclei (often aggregated to form a single group of chromosomes) (Fig. 6G), the haploid female pronucleus (Fig. 6F) and a single needle-shaped nucleus (Fig. 6C). The merged view of DROP 1.1- and DAPI-staining clearly showed that the needle-like nucleus was a sperm nucleus (Fig. 6, C–E), indicating failure of nuclear decondensation. This phenotype was identical to the defect of male sterile mutations sneaky (snky, Fitch and Wakimoto, 1998) and misfire (mfr, Ohsako et al., 2003). Like these two mutations, wst sperm heads in the inseminated eggs were always found near the surface of eggs (data not shown). These observations showed that wst sperm, even when they could enter eggs, failed to undergo nuclear decondensation, which prevented the normal process of fertilization, and ultimately resulted in extremely low hatchability.
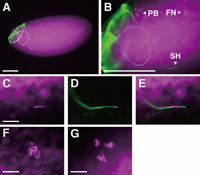 View Details | Fig. 6 Egg inseminated by wst sperm. DAPI and DROP 1.1 staining were pseudo-colored magenta and green, respectively. A single wst sperm is frequently found in the anterior half of egg (A), as in wild type fertilization (Karr, 1991). Higher magnification views (B) of eggs stained with DAPI typically show three fluorescent elements: a needle-like shape, which is clearly identified as a sperm head (SH) in merged views (E) of DAPI (C) and DROP 1.1 (D) staining, as well as the female pronucleus (FN), and the polar body nuclei (PB), whose higher magnification views are shown in F and G, respectively. Bars represent 100 μm in A and B, and 10 μm in C, F and G. |
Here we describe the phenotype of a male sterile mutation named wasted (wst). Females mated to mutant males produce very few progeny, although the males produce normal quantities of motile sperm, which are transferred to females by copulation and are stored normally in sperm storage organs. We have identified at least three defects responsible for the sterility of wst males.
First, the rate at which the mutant sperm are depleted from the sperm storage organs of females is entirely different from that of the control: sperm stored in SR were quickly lost during the first 24 hr after copulation, while those stored in Sp were retained for a longer duration. Since sperm stored in the SR are about 70% of the total stored in all storage organs, a sudden decrease in the number of sperm in the SR should affect fertility significantly.
The observation that wst sperm are lost from SR but not Sp implies that the wst gene product is important for some biochemical interaction of sperm and SR that may not occur in Sp. This interpretation seems reasonable, since the SR and Sp are quite different organs in their origins, shapes, and even in their functions (reviewed in Miller, 1950; Fowler, 1973; Pitnick et al., 1999; Bloch Qazi et al., 2003). Sp is thought to be a long-term storage organ from two general observations: sperm stored in the SR and Sp are lost at different rates (Gilbert, 1981; Neubaum and Wolfner, 1999a) and females deprived of spermathecae lost fertility rapidly (Anderson, 1945; Boulétreau-Merle, 1977; Allen and Spradling, 2008). In the present study, however, the control wst/SM1 sperm stored in Sp were lost faster than those in the SR.
Although the numbers of progeny produced by females storing sperm of the wst/SM1 males declined over time after copulation, hatch rates of the eggs were consistently high. This suggests that stored sperm were utilized efficiently and the decline in the number of progeny was associated primarily with reduced egg production by the females. To explain why the number of wst sperm stored in the Sp organ was consistently high, we propose that many sperm released from sperm storage organs into the uterus upon ovulation are collected by the Sp if they are not used for fertilization. Most wst sperm released excessively from the SR would be collected by the Sp. Assuming that the amount of wst sperm released from Sp is regular, we expect that the number of sperm stored in Sp increases, or does not change when it has reached the capacities of the organs. In fact, at five days after copulation, the wst sperm stored in Sp had decreased at the same rate as the control sperm. The number of wst/Df sperm in Sp is likely lower than the number of wst sperm at five days after copulation because wst/Df sperm are depleted from SR earlier than wst sperm. We often observed wst sperm in the ducts of Sp within the first 24 hr after copulation, though it was impossible to determine if they were being released from the Sp or if they were excess sperm being collected from the uterus (data not shown). Since sperm release was regulated efficiently in females mated to control males, virtually no released sperm were collected by the Sp; consequently, the number of sperm stored in Sp might decline over time after mating. This model does not contradict previously reported observations that the sperm stored in Sp seemed to be used after the depletion of sperm stored in SR (reviewed by Bloch Qazi et al., 2003 and references therein).
Rapid loss of wst sperm from the SR was apparently due to loss of regulation of sperm release. Fertilization in D. melanogaster, unlike the majority of other insect species, occurs as monospermy where essentially a single sperm penetrates into the egg (Perotti, 1975; Karr, 1991; Callaini and Riparbell, 1996). Therefore, efficient use of stored sperm requires that only a few sperm—just enough to fertilize an egg—are released. wst sperm have a defect in regulating sperm use and, as a result, as much as one-third of the stored sperm was released at a single ovulation.
In addition, loss of stored sperm was highly correlated with reduced egg-laying, which is known as the ‘sperm effect’ (Manning 1962, 1967; Kalb et al., 1993; Heifetz et al., 2001). Sex Peptide (also known as Acp70A) that is secreted from male accessory glands (Chen et al., 1988) and carried by sperm (Peng et al., 2005) elicits the sperm effect (Chapman et al., 2003; Liu and Kubli, 2003). Therefore, the rapid depletion of stored sperm not only directly but also indirectly influences fertility. In fact, females laid significantly fewer eggs when mated to wst males than control males on the second and subsequent days after copulation. It is interesting to note that sperm stored in Sp do not seem to contribute to the sperm effect, since oviposition of females mated to wst males declined rapidly 24 hr after copulation even though a considerable amount of sperm had been stored in Sp at the time.
Second, the ability of wst sperm to enter eggs decreased during storage. Shortly after copulation, wst sperm entered eggs as efficiently as control sperm. However, the rate decreased to 58% during 22 to 24 hr after copulation. Since sperm were released upon ovulation, the lowered rate of sperm entry was caused by the loss of capability of sperm entry rather than the depletion of stored sperm or a time gap between sperm release and ovulation. This defect contributed to the sterility, although it constitutes a marginal effect.
Third, wst sperm failed to complete fertilization even when they could enter eggs. Only a small fraction (8%) of eggs inseminated by wst sperm initiated mitotic divisions. The needle-like shape of inseminating wst sperm indicated they failed to undergo nuclear decondensation, which prevented the normal process of fertilization.
Although no mutation that shows all three defects together has been isolated, several mutations that affect each process have been reported. Some enzymes are involved in utilization of stored sperm. First, natural genetic variation in the Est-6 gene, which encodes a component of seminal fluid, affects the release rate of stored sperm (Gilbert, 1981). Females released sperm from males homozygous for a null allele (Est-60) significantly more slowly than sperm from Est-6S homozygotes. The defect shown in Est-60 homozygous males is not severe, so the allele could persist as a polymorphism in natural populations. Second, Glucose dehydrogenase (Gld) is expressed in a subset of reproductive organs in both sexes including Sp (Schiff et al., 1992). Like females mated to Est-60 males, females homozygous for a null Gld mutation used stored sperm at a slower rate over a longer duration than wild-type females (Iida and Cavener, 2004). Despite this, Gld mutant females produced 41% more progeny on average than wild-type females and were thus rather highly fertile. Third, Sex Peptide, a non-enzyme seminal fluid component, also affects sperm release. Females mated to males deficient for Sex Peptide also retained stored sperm for a longer duration (Liu and Kubli, 2003; Avila et al., 2010). This is very likely due to lack of stimulation of ovulation by Sex Peptide, indicating a close relationship between sperm release and ovulation (Liu and Kubli, 2003; Avila et al., 2010). An additional five Acps were shown to be involved in the release of stored sperm. By using RNA interference (RNAi) to knock down levels of individual Acps, four Acp genes (CG1652, CG1656, CG17575 and CG9997) were shown to be necessary for the release of stored sperm (Ravi Ram and Wolfner, 2007). Females mated to these knockdown males stored significantly more sperm in the SR at 10 days after copulation, whereas sperm stored in the Sp were released normally (Ravi Ram and Wolfner, 2007). These phenotypes are opposite to that of wst. Recently, an Acp29AB loss-of-function mutation was shown to cause faster loss of stored sperm, although the loss is mild and there is virtually no effect on fertility (Wong et al., 2008).
The casanova (csn) mutation shows a failure of sperm entry into the egg (Perotti et al., 2001). Two glycosidases, β-N-acetylglucosaminidase and α-D-mannosidase, localized on the plasma membrane of sperm are suggested to have important roles in sperm-egg recognition (Cattaneo et al., 1997; Pasini et al., 1999). The csn sperm lacks β-N-acetylglucosaminidase on the plasma membrane covering the acrosome, suggesting that this prevents sperm from entering the egg (Perotti et al., 2001). This phenotype is clearly different from wst because wst sperm could penetrate efficiently into the egg immediately after copulation. The wst defect is related to the maintenance of sperm entry capability or loss of continuous activation. No such a mutation has ever been reported.
Two fertilization defective mutations, snky (Fitch and Wakimoto, 1998; Wilson et al., 2006) and mfr (Ohsako et al., 2003; Smith and Wakimoto, 2007), show the same nuclear decondensation phenotype as wst mutants, although the storage of sperm and sperm entry into eggs are normal. The sperm heads are always on or near to the inner surface of eggs and retain a needle-like shape morphologically identical to mature sperm. snky and mfr mutant males are likely to be sterile because the sperm fail to form a male pronucleus due to lack of degradation of the sperm plasma membrane. This explanation is possibly applicable to wst mutants as well.
While each wst defect is a deficit in the proper utilization of stored sperm, they affect the extremely diverse processes of the release of stored sperm, sperm entry into the egg, and formation of male pronucleus. It is unlikely that the chromosome giving rise to the wst phenotypes has mutations in more than one gene, because wst males hemizygous with a small deficiency chromosome showed all three defects and male sterile mutations that produce motile sperm capable of transferring to females are very rare (Castrillon et al., 1993; Wakimoto et al., 2004). Instead, wst should be a single mutation. Because the wst phenotype is more severe in hemizygous males than homozygous males, the wst mutation is likely a hypomorphic mutation rather than a null mutation.
We suggest that the wst+ gene is required for modifying sperm when they are stored in SR. The sperm received by copulation may be processed (or activated) in the uterus and sperm storage organs and this proteomic reaction may be essential for subsequent processes in fertilization. In mammals, for example, ejaculated sperm need to be activated in the female genital tract in a process termed capacitation (reviewed in de Lamirande et al., 1997). Capacitation involves molecular changes in both the sperm head and tail, such as motility hyperactivation to produce a whiplash-like sperm tail motion, and regulated acrosomal exocytosis. The motility of wst sperm in SR is as indistinguishable from wild type sperm, but it is possible that sperm must be modified in other ways by female reproductive organs in order to be released properly, pass through the small hole of the micropyle, and migrate to appropriate positions in the egg so that male pronuclear formation can take place. This process could well be the equivalent of capacitation in mammalian fertilization. Several gene products, including a small peptide contained in seminal fluid (Fraser, 1998) and a putative cation channel located specifically in the principal piece of the sperm tail (Ren et al., 2001), have been associated with capacitation. It is possible that the wst gene encodes such a product. Relatively little is known about the mechanism regulating efficient use of stored sperm, especially about molecules essential for this process. The wst mutation is unique because it affects diverse processes in the utilization of stored sperm, implying the presence of a common mechanism, probably sperm modification, during sperm storage. We anticipate that identification and characterization of the wst gene will provide important information on the mechanism of stored sperm utilization.
We thank Stephen DiNardo, Thomas C. Kaufman, Ansgar Santel, Eyal D. Schejter and Chao-ting Wu, the Bloomington Stock Center and the Drosophila Genetic Resource Center, Kyoto, for providing stocks. We also thank Kevin R. Cook for helpful comments on the manuscript, Timothy L. Karr for providing DROP 1.1 antibody, and Suzuko Ohsako for dedicated technical assistance. This study was partly supported by National BioResource Project “Functional Technology Development Program” from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
|