2020 年 95 巻 6 号 p. 281-289
2020 年 95 巻 6 号 p. 281-289
Photoreactivation is a mechanism in which photolyase directly repairs either cyclobutane pyrimidine dimers (CPDs) or (6-4) photoproducts [(6-4) PPs] caused by ultraviolet (UV) light. In the filamentous fungus Neurospora crassa, some UV-sensitive mutants such as mus-44 have been reported to exhibit a partial photoreactivation defect (PPD) phenotype, but its mechanism has not been elucidated for a long time. In this study, the N. crassa CPD photolyase PHR was overexpressed in the Δmus-44 strain, but photoreactivation ability was not increased. Furthermore, Escherichia coli CPD photolyase or Arabidopsis thaliana (6-4) PP photolyase was also introduced into Δmus-44; however, the PPD phenotype was not complemented. These results suggested that the PPD phenotype in N. crassa is not caused by residual unrepaired pyrimidine dimers, which are the main type of DNA damage caused by UV irradiation. Finally, we revealed that Δmus-44, but not the Δmus-43 strain, which does not show the PPD phenotype, displayed higher sensitivity with increasing dose rate of UV. Moreover, Δmus-44 was also sensitive to an interstrand crosslinking agent. This indicates that the high dose of UV in our experimental condition induces DNA damage other than pyrimidine dimers, and that such damage is a likely cause of the PPD phenotype.
DNA, which is the genetic information of life, is continuously damaged in the environment. Failure to repair damaged DNA leads to mutations and cell death. Therefore, all organisms have acquired various DNA repair mechanisms to deal with this damage (Jackson and Bartek, 2009). Ultraviolet (UV) light is a representative exogenous source of DNA damage and induces cyclobutane pyrimidine dimers (CPDs) and (6-4) photoproducts [(6-4) PPs] by covalent bonding of adjacent pyrimidine bases (Sinha and Häder, 2002). In many organisms, these types of damage are repaired by nucleotide excision repair (NER), and the repair mechanism is highly conserved from bacteria to higher eukaryotes. NER-related genes have been isolated in Neurospora crassa, such as mus-38 (a homolog of Saccharomyces cerevisiae RAD1), mus-40 (RAD2), mus-43 (RAD14) and mus-44 (RAD10) (Hatakeyama et al., 1998; Sato et al., 2008; S. Hatakeyama et al., unpublished data). After the publication of the whole genome sequence, it became clear that many other NER-related genes are also conserved (Galagan et al., 2003; M. Sato et al., unpublished data).
In addition to NER, some organisms, such as N. crassa and Schizosaccharomyces pombe, have another excision repair pathway for UV damage, which is UV damage excision repair (UVER) by UV damage-specific endonuclease (UVDE) (Yasui and McCready, 1998). A mutant of the mus-18 gene that encodes UVDE is exclusively sensitive to UV, and double mutants of mus-18 and NER-related genes show hypersensitivity to UV compared to single mutants (Ishii et al., 1991). Therefore, N. crassa and S. pombe are more resistant to UV than S. cerevisiae, which lacks UVER.
One more important mechanism for UV damage repair is photoreactivation. Photoreactivation is a mechanism in which photolyase cleaves pyrimidine dimers directly using light energy from near-UV to blue light, and various types of photolyases are conserved in many organisms, including N. crassa (Thompson and Sancar, 2002). Each photolyase specifically repairs CPDs or (6-4) PPs. Neurospora crassa has a CPD photolyase encoded by phr, but (6-4) PP photolyase is absent (Shimura et al., 1999). Although photolyase is thought to function independently of other repair systems, some mutants such as the NER-related genes mus-38 and mus-44 show a partial photoreactivation defect (PPD) phenotype in N. crassa, the cause of which has not been elucidated for 50 years (Tuveson and Mangan, 1970; Ishii and Inoue, 1989; Sakai et al., 2003).
In this study, to elucidate the cause of the PPD phenotype, N. crassa PHR was overexpressed in the Δmus-44 strain. Furthermore, Escherichia coli Phr (EcPhr), which is a CPD photolyase, and Arabidopsis thaliana UVR3 (AtUVR3), which is a (6-4) PP photolyase, were also expressed, but the reduced photoreactivation ability of the Δmus-44 strain was not complemented. Because N. crassa has hyperresistance to UV, high doses of UV irradiation are required for testing viability among mutants in the experimental condition. Moreover, the Δmus-44 strain showed higher sensitivity with increasing UV dose rate, unlike the wild-type and Δmus-43 strains. This indicates that MUS-44 participates in a repair pathway for another type of DNA damage caused by a high dose rate of UV. Indeed, we found that the Δmus-44 strain showed hypersensitivity to the interstrand crosslink (ICL) agent 1,2,7,8-diepoxyoctane (DEO), but the Δmus-43 strain did not.
These results suggest that the PPD phenotype of N. crassa is not due to unrepaired pyrimidine dimers. A potential cause of the PPD phenotype is another type of damage, perhaps ICLs, generated experimentally by a high dose of UV irradiation.
Neurospora crassa strains used in this study are listed in Table 1. C1-T10-37A and C1-T10-28a are wild-type strains that are closely related to the standard Oak Ridge wild-type strains (Tamaru and Inoue, 1989). General genetic manipulations, including crosses, were carried out as described by Davis and de Serres (1970). Vogel’s minimal medium (Vogel’s salts with 1.2% sucrose and 1.2% agar) was used for general culture. Colony formation medium (Vogel’s salts with 1% sorbose, 0.05% glucose, 0.05% fructose and 1.2% agar) was used for colony formation assay. To use the his-3 mutants, histidine (final concentration of 0.5 mg/ml) was added to all media.
| Strain | Genotype | Source, reference |
|---|---|---|
| C1-T10-37A | A | Tamaru and Inoue (1989) |
| C1-T10-28a | a | Tamaru and Inoue (1989) |
| FGSC 2356 | A his-3 | FGSCa |
| FGSC 18983 | a phr::Hygr | FGSC |
| FGSC 12166 | a mus-43::Hygr | FGSC |
| FGSC 12165 | A mus-44::Hygr | FGSC |
| TSKD-001 | a mus-44::Hygr | This study |
| TSKD-002 | A phr::Hygr his-3 | This study |
| TSKD-006 | A mus-43::Hygr his-3 | This study |
| TSKD-008 | A mus-44::Hygr his-3 | This study |
| TSKD-011 | A mus-43::Hygr phr::Hygr his-3 | This study |
| TSKD-012 | A mus-44::Hygr phr::Hygr his-3 | This study |
| TSKD-013 | A his-3+:Pphr-3×FLAG-Ncphr | This study |
| TSKD-023 | A phr::Hygr his-3+:Pphr-3×FLAG | This study |
| TSKD-014 | A phr::Hygr his-3+:Pphr-3×FLAG-Ncphr | This study |
| TSKD-016 | A mus-44::Hygr his-3+:Pphr-3×FLAG-Ncphr | This study |
| TSKD-018 | A mus-44::Hygr phr::Hygr his-3+:Pphr-3×FLAG-Ncphr | This study |
| TSKD-019 | A phr::Hygr his-3+:Pphr-3×FLAG-Ecphr | This study |
| TSKD-020 | A mus-43::Hygr phr::Hygr his-3+:Pphr-3×FLAG-Ecphr | This study |
| TSKD-021 | A mus-44::Hygr phr::Hygr his-3+:Pphr-3×FLAG-Ecphr | This study |
| TSKD-030 | A phr::Hygr his-3+:Pccg-1-3×FLAG | This study |
| TSKD-024 | A phr::Hygr his-3+:Pccg-1-3×FLAG-Ncphr | This study |
| TSKD-025 | A mus-44::Hygr phr::Hygr his-3+:Pccg-1-3×FLAG-Ncphr | This study |
| TSKD-031 | A phr::Hygr his-3+:Pccg-1-3×FLAG-AtUVR3 | This study |
| TSKD-032 | A mus-43::Hygr his-3+:Pccg-1-3×FLAG-AtUVR3 | This study |
| TSKD-033 | A mus-44::Hygr his-3+:Pccg-1-3×FLAG-AtUVR3 | This study |
pFLAGN1 plasmid, which was used as a vector for his-3 targeted transformation, carries the 5′-truncated his-3 gene of N. crassa, and an ampicillin resistance gene (Ampr) as a selection marker in E. coli (Kawabata and Inoue, 2007). Escherichia coli strain DH5α was used for amplification of plasmids.
The N. crassa phr gene was amplified from genomic DNA of wild type by primers phr_FLAG_F and phr_FLAG_R (Supplementary Table S1). The amplicon was cloned into pFLAGN1 amplified with primers pFLAGN1_ApaI_F and pFLAGN1_SmaI_R by In-Fusion (Takara Bio) to construct pTSKD1 (Supplementary Fig. S1A). Next, a PCR amplicon generated from genomic DNA of wild type using primers phr_promoter_F and phr_promoter_R was introduced into pTSKD1 amplified with pFLAGN1_XbaI_F and pFLAGN1_NotI_R by In-Fusion to construct pTSKD2 (Supplementary Fig. S1B). As control vectors, pTSKD1 and pTSKD2 were treated with ApaI and BamHI, respectively, and T4 DNA polymerase, to remove the phr gene. After being electrophoresed, the target bands were extracted and self-ligated to construct pTSKD3 and pTSKD4, respectively (Supplementary Fig. S1C and S1D).
The E. coli phr gene was amplified from DH5α genomic DNA using primers Ecoli_phr_F and Ecoli_phr_R. The amplicon was introduced into pTSKD2 amplified by primers pFLAGN1_ApaI_F and pFLAGN1_SmaI_R by In-Fusion to construct pTSKD5 (Supplementary Fig. S1E).
For cloning of the AtUVR3 gene, Arabidopsis thaliana cDNA was synthesized from total RNA. Reverse transcription was performed with oligo(dT) (20 nt) primers using ReverTra Ace (Toyobo). AtUVR3 cDNA was amplified from total cDNA using primers AtUVR3_if_F and AtUVR3_if_R. The amplicon was introduced into pTSKD1 amplified by primers pFLAGN1_ApaI_F and pFLAGN1_SmaI_R by In-Fusion to construct pTSKD6 (Supplementary Fig. S1F).
Transformation of N. crassaNeurospora crassa transformation was performed by electroporation as described previously with slight modifications (Margolin et al., 1997; Ninomiya et al., 2004). A conidial suspension was washed with 1 M sorbitol three times, and linearized plasmids (more than 300 ng) were then added to 40 μl of the suspension and cooled on ice for 5 min. The total conidial suspension containing plasmids was then transferred to a chilled electroporation cuvette (2 mm width). Electroporation was performed using the ECM 630 Electroporation System (BTX) set at 1.5 kV, 200 Ω, and 50 μF. After electroporation, 960 μl of 1 M sorbitol was added to the cuvette, and the suspension was mixed by gently pipetting, transferred to a new micro tube, and incubated on ice for 10 min. The conidial suspension was then mixed with top agar (Vogel’s salts with 2% sorbose, 1 M sorbitol and 1.5% agar), and spread over colony formation medium. Transformants were picked after 2–3 days of incubation at 30 ℃. his-3-targeted homokaryotic cells were obtained after single-colony isolation. Homokaryons were verified with PCR using primers his-3_3’_flank_F and his-3_terminal_R.
UV irradiation and photoreactivationQuantitative sensitivity to UV was measured by colony formation assay as described previously (Inoue and Ishii, 1984). A 20-ml conidial suspension (1 × 106 conidia/ml in 0.067 M phosphate buffer, pH 7.0) was irradiated using a germicidal lamp at 254 nm (Toshiba, GL10). The dose rate was at 2.0 J/m2/s, except that part of the experiment in Fig. 5 was 2.5 J/m2/s (see Results). After irradiation, 1 × 103 conidia were mixed with colony formation medium, and then poured into a Petri dish (diameter of 15 cm). Each plate was incubated for two days at 30 ℃ and the numbers of colonies were counted. To avoid unintended photoreactivation, UV irradiation and incubation were conducted in the dark or under red light. The average of two plates for each dose was determined, and survival rates were calculated from an unirradiated control. For photoreactivation assay after UV irradiation, remaining conidial suspension was illuminated with white light for 60 min in a growth chamber (Nippon Medical & Chemical Instruments, LH-60FL12-DT).
Spot testQualitative sensitivity to chemical mutagens was measured by spot test. Each mutagen at the indicated concentrations was added to colony formation medium. Conidia were suspended at a concentration of 1 × 106 conidia/ml, diluted by 1/4 serial dilution, and 10 μl of each suspension was spotted on the medium. They were incubated at 30 ℃ for two days.
Protein isolation and Western blottingA suspension of 1 × 107 conidia/ml was UV-irradiated at 200 J/m2 and an aliquot was illuminated with white light for 60 min as described above. The conidia were harvested by centrifugation, and total cell protein was then extracted using protein isolation buffer [50 mM HEPES buffer, pH 7.6, 10% glycerol, 137 mM NaCl, cOmplete protease inhibitor cocktail (Roche Diagnostics)] by homogenizing in a Micro Smash MS-100 (Tomy). The extracts were electrophoresed in 10% SDS-PAGE gels and transferred onto a PVDF membrane. After being washed in TBST (25 mM Tris-HCl, pH 7.4, 137 mM NaCl, 2.68 mM KCl and 0.2% Tween 20) buffer three times, the membrane was blocked using blocking buffer (0.3% skim milk in TBST buffer) for 1 h at room temperature and then probed with anti-FLAG antibody (1/5,000, Wako, 011-22393) in 10 ml blocking buffer for 2 h at room temperature. After the probed membrane was washed in TBST buffer three times, it was treated with chemiluminescent substrate (EzWestLumi plus, ATTO, WSE-7120S). FLAG-tagged proteins were detected using ChemiDoc XRS (Bio-Rad) according to the manufacturer’s direction.
Detection of UV-induced CPDs by T4 endonuclease VA suspension of 2.5 × 107 conidia/ml was UV-irradiated at 300 J/m2 as described above. The suspension was divided into two centrifuge tubes, and one of them was illuminated with white light in the same manner as the photoreactivation method. After centrifuging and collecting the conidia, genomic DNA was extracted with 500 μl of isolation buffer [5 mM Tris-HCl, pH 8.0, 170 mM EDTA, pH 8.0, 1% N-lauroylsarcosine] and treated with T4 endonuclease V (New England Biolabs) overnight at 37 ℃. The treated samples were electrophoresed in a 1% denaturing alkaline agarose gel at a constant 25 V for 12 h, and the gel was stained with ethidium bromide to visualize the DNA.
The mus-43 and mus-44 genes in N. crassa are homologs of S. cerevisiae RAD14 and RAD10, respectively. Their gene products are considered to be involved in NER, even in N. crassa, as mutants of these genes are sensitive to UV and 4-nitroquinoline 1-oxide (4NQO) and exhibit an epistatic relationship with the RAD1 homolog mus-38 (Sato et al., 2008). In this study, deletion strains of these genes produced by the KO project of the Fungal Genetics Stock Center were used. Δmus-43 and Δmus-44 strains showed greater UV sensitivity than the wild type (Fig. 1A and 1B). Also, the Δmus-44 strain was more sensitive to UV than the Δmus-43 strain (Fig. 1B). These results were similar to mutants produced by repeat-induced point mutation (Sato et al., 2008). Furthermore, to evaluate the photoreactivation ability of these strains, a photoreactivation assay was performed in which conidia were irradiated with visible light for 60 min after irradiation with UV light of each dose. The photoreactivation ability of the Δmus-43 strain was comparable to that of the wild-type (Fig. 1A). However, the Δmus-44 strain showed a more remarkable, although not complete, loss of photoreactivation ability than the wild-type and Δmus-43 strains (Fig. 1B).
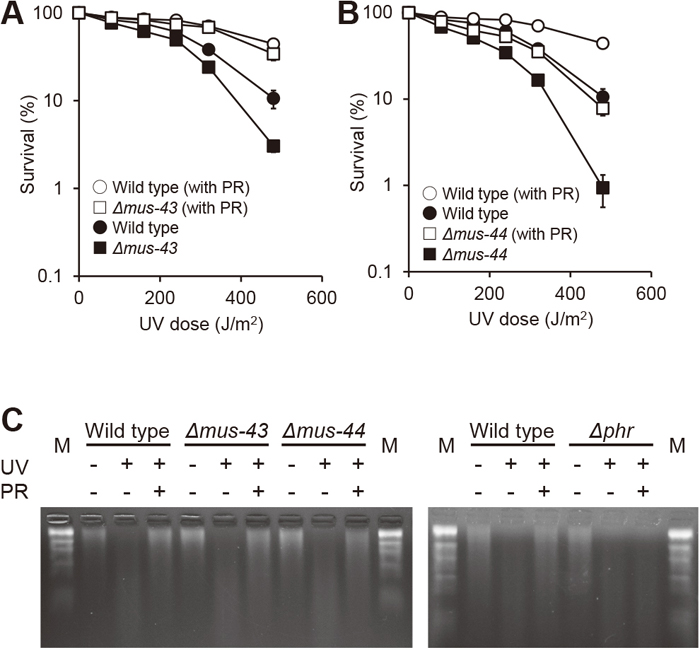
UV sensitivity and photoreactivation of Δmus-43 and Δmus-44 strains. (A and B) Conidial suspensions were UV-irradiated with the indicated dose at 2.0 J/m2/s and immediately plated with medium or light-illuminated for 60 min before plating for photoreactivation (PR). After incubation for two days, colonies in each dish were counted. Error bars indicate standard errors calculated from data of at least three independent experiments. Each dose was calculated by irradiation time depending on the indicated dose rate. For example, at 100 J/m2 UV dose, a rate of 2.0 J/m2/s requires 50 s and a rate of 2.5 J/m2/s requires 40 s of UV irradiation. (C) Detection of UV-induced CPDs by T4 endonuclease V. Immediately after 300 J/m2 UV irradiation (UV) or 60 min visible light illumination (PR), conidial DNA was extracted, treated with T4 endonuclease V and electrophoresed in a 1% denaturing alkaline agarose gel. M: DNA size marker (λ HindIII digest).
To directly compare the amount of repaired CPDs after photoreactivation, we conducted another experiment using T4 endonuclease V, which functions as a glycosylase at CPD sites (Nakabeppu et al., 1982). Based on this principle, we measured CPD repair abilities after UV irradiation and photoreactivation by electrophoresis under denaturing conditions in an alkaline agarose gel. In all strains indicated in Fig. 1C including Δmus-44, CPDs were almost completely repaired by a 60-min photoreactivation treatment, resulting in a migration pattern similar to that of the unirradiated control. On the other hand, in the Δphr strain, we confirmed that the migration pattern after the photoreactivation treatment was not changed compared to that before photoreactivation (Fig. 1C). These results indicate that most of the CPD repair activity during photoreactivation for 60 min depends on the photolyase.
These results, together with a previous report (Sato et al., 2008), suggested that some cause of functional loss of MUS-44 protein resulted in the PPD phenotype.
Overexpression of PHR from the ccg-1 promoter does not complement the PPD phenotype of the Δmus-44 strainTo investigate under what conditions the PPD phenotype of Δmus-44 can be compensated for, a plasmid was constructed to overexpress N. crassa photolyase PHR under the ccg-1 (formerly grg-1, McNally and Free, 1988) promoter, which is used as a general forced expression promoter in N. crassa, and introduced at the his-3 locus. As a control strain, the 1 kbp immediately upstream of phr was used as a promoter region and also introduced at the his-3 locus. The photoreactivation ability of 3×FLAG-tagged PHR was normal in the Δphr strain (Fig. 2A). Resistance to UV light was also increased in the PHR-overexpressing strain. It was possible that photoreactivation was caused by the slightly blue light from the UV lamp due to the effect of the ccg-1 promoter. As a result of overexpression of PHR in the Δmus-44 Δphr double-knockout strain, no difference was observed in photoreactivation ability upon PHR expression from either the phr or the ccg-1 promoter (Fig. 2B). Compared to the values in Fig. 2A, the PPD phenotype of the Δmus-44 strain was obviously observed in both strains.

Effects on PPD of overexpressing PHR under the ccg-1 promoter. (A and B) UV sensitivity and photoreactivation assays were performed as described in Fig. 1. Neurospora crassa PHR was expressed under the endogenous phr promoter (Pphr-phr) or the ccg-1 promoter (Pccg-1-phr). Each value was analyzed and is shown as described in Fig. 1. (C) Detection of 3×FLAG-tagged PHR. Proteins from light-illuminated conidia after UV irradiation were analyzed by Western blotting. Lane 1, wild type; lanes 2 to 5, strains expressing PHR under the endogenous phr promoter in the indicated genetic background; lanes 6 and 7, strains expressing PHR under the ccg-1 promoter in the indicated background.
To confirm the expression level of PHR, proteins were extracted from conidia that had been subjected to illumination with white light after UV irradiation at 200 J/m2, and Western blotting was performed using an anti-FLAG antibody. With regard to expression from the phr promoter, the expression level of PHR was decreased in the Δphr and Δmus-44 strains (Fig. 2C, lanes 3 and 4) compared to the wild type (Fig. 2C, lane 2). Furthermore, the Δmus-44 Δphr strain showed an additive decrease in the expression of PHR (Fig. 2C, lane 5). The reason for this may be that as N. crassa PHR is induced by light (unpublished data), damage to the phr gene region by UV irradiation was not repaired due to the deficiency of repair proteins PHR and MUS-44. In contrast, the overexpression under the ccg-1 promoter did not result in any decrease in PHR expression in the Δmus-44 Δphr strain and was similar to the Δphr strain (Fig. 2C, lanes 6 and 7).
These results suggested that the PPD phenotype is not due to a decrease in the repair activity of PHR, as the decreased photoreactivation ability of the Δmus-44 strain was not complemented even if the expression of PHR was increased.
Escherichia coli CPD photolyase does not complement the PPD phenotype of the Δmus-44 strainOverexpression of N. crassa PHR did not complement the PPD phenotype of the Δmus-44 strain, raising the possibility that PHR is involved in the removal of CPDs by cooperating with MUS-44 and other dark repair systems. Therefore, we examined whether the photoreactivation ability was compensated for by introducing another photolyase derived from a quite different species, E. coli photolyase (EcPhr), which repairs CPDs, like N. crassa PHR. When 3×FLAG-tagged EcPhr was expressed in the Δphr strain under the control of the Ncphr promoter, survival after photoreactivation increased slightly compared to the vector control strain (Fig. 3A), thus confirming that EcPhr can function in N. crassa. When EcPhr was expressed in the Δmus-43 Δphr strain, survival after photoreactivation also increased (Fig. 3B). In contrast, the Δmus-44 Δphr strain showed only slight photoreactivation ability (Fig. 3C). Analysis of EcPhr expression by Western blotting revealed that the expression level was approximately the same in Δmus-43 and Δmus-44 background strains (Fig. 3D). These results indicated that EcPhr did not complement the PPD phenotype of the Δmus-44 strain.
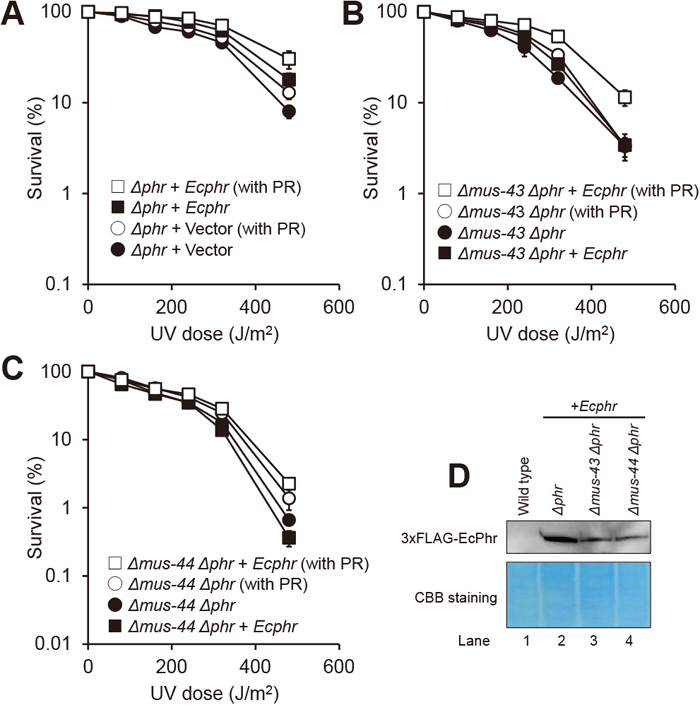
Effects on PPD of introducing photoreactivation by EcPhr. (A to C) UV sensitivity and photoreactivation assays were performed, analyzed and presented as described in Fig. 1. EcPhr was expressed under the endogenous phr promoter. To compare photoreactivation ability, empty vector control (A) or parental strains of transformation (B and C) are shown in the same graph. (D) Detection of 3×FLAG-tagged EcPhr. Western blotting analysis was performed as described in Fig. 2. Lane 1, wild type; lanes 2 to 4, strains expressing EcPhr in the indicated genetic background.
The most important direct effect of UV on DNA is the formation of dimers between adjacent pyrimidines, mainly CPDs and to a lesser extent (6-4) PPs (Friedberg et al., 2006). There was a possibility that the PPD phenotype is caused by (6-4) PPs. Therefore, we investigated whether AtUVR3, which is a (6-4) PP photolyase (Nakajima et al., 1998), can complement the PPD phenotype when introduced into the Δmus-44 strain. Expression of AtUVR3 in N. crassa was expected to generate an abundant quantity of (6-4) PP photolyase. When AtUVR3 was expressed under the ccg-1 promoter, the photoreactivation ability of the Δphr strain was higher than that of the vector control, indicating that AtUVR3 can function in N. crassa (Fig. 4A). However, in Δmus-43 and Δmus-44 strains, there was no additive increase in the survival rate after photoreactivation (Fig. 4B and 4C). These results suggested that (6-4) PP is less detrimental to cell survival than CPD and hardly appears in the phenotype after repair of CPD with N. crassa PHR. In addition, the expression level of AtUVR3 in these strains was very similar regardless of the presence or absence of an NER-deficient background (Fig. 4D). From these results, the impact of UV-induced (6-4) PP on both UV sensitivity and PPD phenotype was smaller than that of CPD, and AtUVR3 did not complement the photoreactivation ability of Δmus-44. Therefore, we concluded that the PPD phenotype is not caused by residual (6-4) PPs.

Effects on PPD of introducing photoreactivation by A. thaliana UVR3. (A to C) UV sensitivity and photoreactivation assays were performed, analyzed and presented as described in Fig. 1. Arabidopsis thaliana UVR3 was expressed under the ccg-1 promoter. To compare the photoreactivation ability, empty vector control (A) or parental strains of transformation (B and C) are shown in the same graph. (D) Detection of 3×FLAG-tagged AtUVR3. Western blotting analysis was performed as described in Fig. 2. Lane 1, wild type; lanes 2 to 4, strains expressing AtUVR3 in the indicated genetic background.
UV causes various types of damage, whose repair involves many repair pathways (Rastogi et al., 2010). In experimental conditions, UV irradiation is performed at a constant dose rate, and the dose is calculated based on the irradiation time. In the next experiment, we increased the dose rate from 2.0 to 2.5 J/m2/s to perform a more acute treatment (Fig. 5A). No difference in survival was observed in both the wild-type and Δmus-43 strains at the respective dose rates. However, Δmus-44 was more sensitive at the higher dose rate of 2.5 J/m2/s.

Function of MUS-44 in NER-independent repair pathways. (A) UV sensitivity assay was performed, analyzed and presented as described in Fig. 1, except that the dose rate was increased from 2.0 to 2.5 J/m2/s. (B) Sensitivity of Δmus-43 and Δmus-44 strains to various mutagens. Conidia suspensions were spotted on colony formation medium containing the indicated concentrations of agents, and incubated for two days. Drugs: 4NQO, 4-nitroquinoline 1-oxide; DEO, 1,2,7,8-diepoxyoctane; BLM, bleomycin; CPT, camptothecin; MMS, methyl methanesulfonate; HU, hydroxyurea.
The mus-44 gene product is involved in NER but has not been implicated in other repair pathways. However, since homologous genes of other organisms are known to be involved in various repair pathways, we analyzed the involvement of MUS-44 in repair pathways other than NER. 4NQO distorts the DNA double helix by producing bulky adducts in DNA and is used as a UV-mimic agent. DEO is an ICL agent that forms covalent crosslinks between DNA strands. Bleomycin causes DNA double-strand breaks. Camptothecin, as an inhibitor of topoisomerase I, causes DNA–protein crosslinks and single-strand breaks. Methyl methanesulfonate causes alkylation of DNA, while hydroxyurea inhibits replication by depleting dNTPs in cells. Both Δmus-43 and Δmus-44 strains were equally sensitive to 4NQO, and more so than wild type, while Δmus-44 showed hypersensitivity to DEO, unlike Δmus-43 (Fig. 5B).
These results suggest that MUS-44 is involved in an NER-independent repair pathway that responds to damage caused by UV irradiation with a high dose rate, which may induce ICLs.
Half a century has passed since it was first reported that some UV-sensitive mutants of N. crassa show decreased photoreactivation ability (Tuveson and Mangan, 1970). However, the genes responsible for the PPD phenotype are not those encoding photolyases (Shimura et al., 1999). In addition, as no such PPD phenotype has been reported in other organisms, the phenomenon is considered to be specific to N. crassa. Therefore, N. crassa was thought to have unique relationships between photoreactivation and other repair systems, but the mechanism underlying such relationships remains unclear. In this study, we found that the PPD phenotype was not complemented by the overexpression of N. crassa PHR in one of the PPD strains, Δmus-44. Furthermore, forced expression of two heterologous photolyases, EcPhr and AtUVR3, could not complement the PPD phenotype of Δmus-44. These results suggested that the PPD phenotype was not caused by two types of pyrimidine dimers, namely CPD and (6-4) PP produced by UV.
In a previous analysis, reverse transcription-PCR revealed that the expression level of phr in Δmus-44 was not different from that in Δmus-43, which does not show the PPD phenotype, suggesting that PPD is not due to decreased expression of phr (unpublished data). In this study, even though 3×FLAG-PHR under the control of either the endogenous phr promoter or the overexpressing ccg-1 promoter was ectopically expressed in Δmus-44 with the Δphr background, no increase of photoreactivation ability was observed (Fig. 2B). These results suggested that the PPD phenotype exhibited by Δmus-44 does not depend on the expression level of PHR. Because photoreactivation by photolyase is generally a fast one-enzyme process (Sancar, 2000), CPDs may be completely removed after 60 min (Fig. 1A).
In S. cerevisiae, photolyase Phr1 reportedly works in cooperation with the NER-related protein Rad2 and participates in NER (Sancar and Smith, 1989). For this reason, it seemed possible that MUS-44 and PHR function in the same pathway, and thus that photoreactivation by photolyases derived from different organisms might be performed normally. However, the photoreactivation ability of Δmus-44 did not increase in the presence of active EcPhr (Fig. 3C). This suggested that N. crassa PHR functions independently and that the photoreactivation abnormality due to mus-44 deficiency is not caused by the decrease in CPD photolyase function.
Neurospora crassa PHR specifically removes CPD but does not work against (6-4) PPs (Shimura et al., 1999). In contrast, diverse organisms such as Drosophila melanogaster, Xenopus laevis and A. thaliana were confirmed to contain (6-4) PP photolyase (Todo et al., 1993, 1997; Nakajima et al., 1998). Expression of the introduced AtUVR3, a (6-4) PP photolyase from A. thaliana, was confirmed in N. crassa (Fig. 4D), and we ascertained that AtUVR3 can function in N. crassa (Fig. 4A). However, the photoreactivation ability when AtUVR3 was expressed in Δmus-44 hardly increased (Fig. 4C). The amount of UV-induced CPD was reported to be about five times that of (6-4) PPs in human cells (Lo et al., 2005). In fact, even in A. thaliana, the uvr3 mutant was less sensitive after UV irradiation and photoreactivation than the CPD photolyase uvr2 mutant (Jiang et al., 1997). From these results, it appears that the cause of the PPD phenotype is not (6-4) PPs.
Finally, we revealed that the Δmus-44 strain shows higher sensitivity with increasing dose rate of UV (Fig. 5A). Unlike S. cerevisiae, N. crassa and S. pombe have UVDE, which specifically repairs UV damage independently from NER (Yasui and McCready, 1998). Saccharomyces cerevisiae NER mutants show hypersensitivity, with less than 0.1% survival at only 10 J/m2 UV (Guzder et al., 2006). In contrast, our previous work indicated that N. crassa NER mutants show relatively mild sensitivity to UV, as mus-18, which encodes UVDE, contributes greatly to the repair of pyrimidine dimers (Hatakeyama et al., 1998; Sato et al., 2008). Thus, considering that PPD is a phenotype specific to N. crassa, its cause may be DNA damage other than pyrimidine dimers, and especially damage caused by high doses of UV irradiation. In nature, UV irradiation from sunlight is ~0.0017 J/m2/s, a moderate dose rate (Callegari and Kelly, 2006). In contrast, under experimental conditions, a high dose rate of UV is typically irradiated within a short time. Presumably, such acute treatment may induce damage other than pyrimidine dimers, such as DNA–DNA and/or DNA–protein crosslinks, at a high frequency (Rastogi et al., 2010). Indeed, the Δmus-44 strain, but not Δmus-43, showed significant sensitivity to the ICL agent DEO (Fig. 5B). As MUS-44 may be involved in the repair of these types of damage, we consider that the apparent decrease of photoreactivation ability occurs after UV irradiation. Since it is well established that the human XPF–ERCC1 heterodimer (homologs of MUS-38 and MUS-44, respectively) is involved in the ICL repair pathway (McNeil and Melton, 2012), it is conceivable that a MUS-38/MUS-44 heterodimer also regulates incision at the ICL.
In conclusion, we suggest that the PPD phenotype in N. crassa is not caused by CPDs or (6-4) PPs. The phenotype may instead reflect a defect of ICL repair ability in mutant strains.
We thank the Broad Institute and FungiDB for making the N. crassa genome database. We also thank the Fungal Genetics Stock Center for providing Neurospora strains.