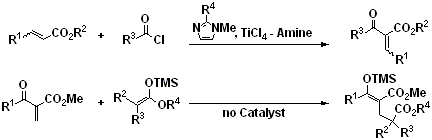34th Symposium on Progress in Organic Reactions and Syntheses
Session ID : 1P-30
Conference information
Host: Division of Organic Chemistry, The Pharmaceutical Society of Japan
AM 11:10~PM 0:22/Poster short talk
Morita-Baylis-Hillman-type reaction utilizing TiCl4-amine reagent
Keywords:
Morita-Baylis-Hillman reaction,
Titanium tetrachloride,
N-methyimidazole,
a,b-unsaturatedester,
ketene silyl acetal
Details