2021 Volume 70 Issue 1 Pages 1-5
2021 Volume 70 Issue 1 Pages 1-5
This study aimed to evaluate the frequency and extent of chemotherapy-induced nausea and vomiting (CINV) in patients with colorectal cancer (CRC) who underwent chemotherapy, using the Multinational Association of Supportive Care in Cancer (MASCC) Antiemesis Tool (MAT), and establish an antiemetic protocol for the management of CINV, independent of the treating oncologist. We conducted a prospective observational study from October 2017 to June 2018 at the Higashihiroshima Medical Center. Patients who had undergone moderate CINV risk chemotherapy for CRC were eligible. The incidence of CINV was evaluated using the MAT, and medical prophylaxis was gradually provided following the antiemetic protocol. We enrolled 30 patients, and 27 of 30 patients (90%) were assessed more than once using the MAT. Among these 27 patients, the incidence of acute nausea was 30% and delayed nausea was 26% as evaluated using the MAT based on recommended pharmacological prophylaxis guidelines. Between the start and end of the survey, there was no significant difference in the numeric rating scale (NRS) score for acute nausea, but there was a significant reduction in the NRS score for delayed nausea. The clinical significance of the antiemetic protocol as assessed using the MAT in reducing CINV was demonstrated. The clinical use of the protocol may help in the realization that selective pharmacological prophylaxis for patients with CINV is possible, independent of the treating oncologist.
Chemotherapy-induced nausea and vomiting (CINV) is one of the most undesirable adverse events caused by cancer therapy, which leads to a poor quality of life and reduced chemotherapy adherence3–5). Inadequate control of CINV can lead to physical disorders, including dehydration, electrolyte abnormalities, and malnourishment, especially in patients with gastroenterological malignancies.
According to some antiemetic guidelines, adequate management of CINV enables patients to complete chemotherapy regimens and maintain quality of life. CINV is classified as either acute or delayed, based on whether it occurs within 24 hr or more than 24 hr after the beginning of chemotherapy. There are some clinical issues in the management of CINV, including medical staff who tend to underestimate the severity and incidence of delayed CINV, the lack of an objective evaluation regarding the extent of acute and delayed CINV, and the lack of a uniform management protocol for CINV for oncologists.
This study aimed to evaluate the frequency and extent of CINV in patients with colorectal cancer (CRC) who underwent chemotherapy at our hospital, as evaluated using the Multinational Association of Supportive Care in Cancer (MASCC) Antiemesis Tool (MAT), and establish an antiemetic protocol for the management of CINV, independent of the treating oncologist.
We conducted a prospective observational study from October 2017 to June 2018 at the Higashihiroshima Medical Center. Patients who had undergone moderate CINV risk chemotherapy for CRC were eligible.
We used the MAT to evaluate the incidence of CINV. The MAT was developed as a subjective patient self-evaluation tool for CINV in 2004 that can be used to evaluate the incidence of CINV6). The MAT is a patient-reported questionnaire that patients complete on the first and fifth days after the beginning of chemotherapy. The MAT is used to assess CINV based on severity level (1 to 10; 10 = most severe) during the first 5 days after chemotherapy (Figure 1).

Multinational Association of Supportive Care in Cancer Antiemesis Tool (MAT).
We provided medical prophylaxis based on the risk classification of emesis by the Japan Society of Clinical Oncology Guidelines for the Optimal Use of Antiemetics (Japanese guidelines)10). After providing oral consent to the procedure, patients received the questionnaires at the beginning of chemotherapy and were requested to respond to questions regarding the status of symptoms occurring within 5 days after treatment. The questionnaires were retrieved after their next treatment and evaluated.
For the initial standard pharmacological prophylaxis, a combination of a first-generation 5-HT3 receptor antagonist (5-HT3RA, granisetron) and dexamethasone was applied in oxaliplatin-based regimens, and a combination of a second-generation 5-HT3RA (palonosetron) and dexamethasone was applied in irinotecan-based regimens; the latter had stronger emetic effects than the oxaliplatin-based regimens, based on the Japanese guidelines. In the evaluation using the MAT, if there were any symptoms of CINV, medical prophylaxis was gradually added to the antiemetic protocol by the attending doctor. The details of the antiemetic protocol are demonstrated in Figure 2. At the discretion of the attending doctor, the use of other antiemetic drugs, such as metoclopramide and antacid, were allowed. The MAT evaluation was continued until the end of treatment (i.e., completion of adjuvant chemotherapy regimen, change in treatment because of disease progression, or death).

Antiemetic protocol. a. Oxaliplatin-based regimen. b. Irinotecan-based regimen.
This study was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent. The institutional review boards or research ethics committees approved the protocol (approval code: 29–53).
Statistical analysesThe statistical significance of differences between the groups was analyzed using the chi-square test and the Wilcoxon t-test. In all analyses, statistical significance was set at P < 0.05. All statistical analyses were performed using the International Business Machines Statistical Package for the Social Sciences (IBM SPSS) software version 20.0.
We enrolled 30 patients from October 2017 to June 2018. There were 22 men and eight women with a median age of 66.5 years (range, 34–78 years). Chemotherapy was provided as adjuvant therapy in eight patients and to treat unresectable advanced disease in 22 patients. The oxaliplatin-based regimen was administered to 18 patients and the irinotecan-based regimen was administered to 12 patients. Combination therapy with molecular targeted drugs was administered to 18 patients (bevacizumab, n = 10; ramucirumab, n = 1; and panitumumab, n = 6).
Chemotherapy was performed in 22 patients as a first-line therapy and in eight patients as a second-line therapy. The total median number of MAT evaluations was 4 (range, 0–6).
Frequency and extent of CINV as evaluated using the MATWe received more than one MAT result from 27 of 30 patients (90%). Among these 27 patients, acute vomiting and delayed vomiting occurred in four patients each (15% and 15%). Acute nausea developed in eight patients (30%) and delayed nausea in seven (26%). There was no significant difference in CINV incidence between patients receiving first-line therapy and second-line therapy (acute vomiting, P = 0.35; acute nausea, P = 0.57; delayed vomiting, P = 0.28; delayed nausea, P = 0.28).
Difference in the incidence of CINV between the oxaliplatin-based (n = 15) and irinotecan-based (n = 12) regimensAcute vomiting occurred in two patients (13%) treated with the oxaliplatin-based regimen and two patients (17%) treated with the irinotecan-based regimen (P = 0.81). Acute nausea occurred in four patients (27%) treated with the oxaliplatin-based regimen and in four patients (33%) treated with the irinotecan-based regimen (P = 0.71). Delayed vomiting occurred in two patients (13%) treated with the oxaliplatin-based regimen and two patients (17%) treated with the irinotecan-based regimen (P = 0.74). Delayed nausea occurred in four patients (27%) treated with the oxaliplatin-based regimen and three patients (25%) treated with the irinotecan-based regimen (P = 0.97). There were no significant differences in the incidence of CINV between the oxaliplatin- and irinotecan-based regimens.
Differences in the incidence of CINV between the first and last surveyAmong the 22 patients who provided questionnaire responses more than twice, the incidence of acute vomiting according to the first and last survey was four cases (18%) (Figure 3a). The incidence of acute nausea according to the first and last survey was seven cases (32%) and eight cases (36%), respectively (Figure 3b). The incidence of delayed vomiting according to the first and last survey was four cases (18%) and two cases (9%), respectively (Figure 3c). The incidence of delayed nausea according to the first and last survey was seven cases (32%) and six cases (27%), respectively (Figure 3d). No significant reduction in CINV was observed.
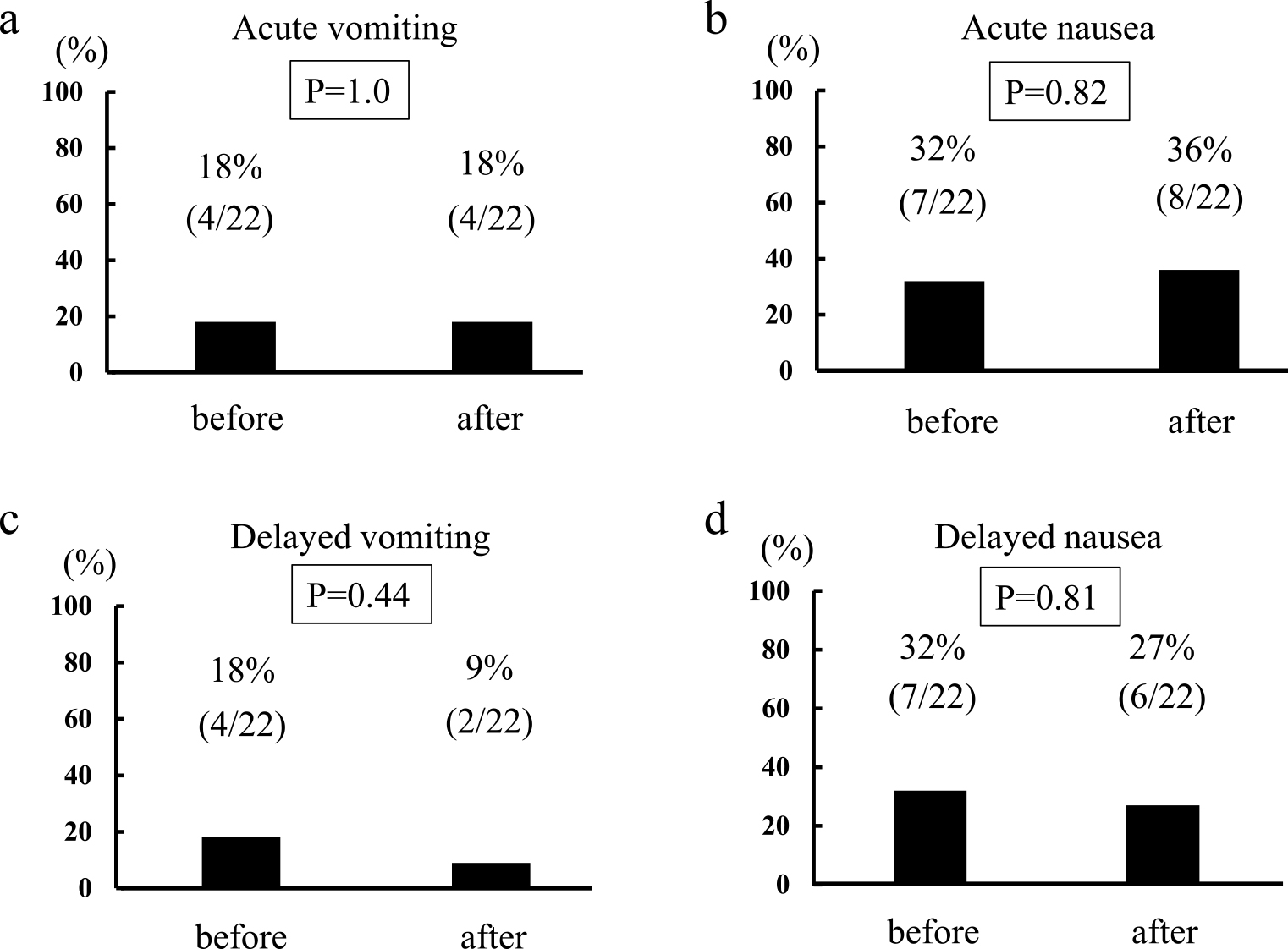
Differences in the incidence of chemotherapy-induced nausea and vomiting (CINV) between the first and last survey. a. Acute vomiting. b. Acute nausea. c. Delayed vomiting. d. Delayed nausea.
Between the first and last survey, there was no significant difference in the numeric rating scale (NRS) score for acute nausea (P = 0.34) (Figure 4a), but there was a significant reduction in the NRS score for delayed nausea (P = 0.02) (Figure 4b). This result indicates the clinical significance of the antiemetic protocol in the reduction of delayed nausea.

Difference in the incidence of chemotherapy-induced nausea and vomiting (CINV) as evaluated using the numeric rating scale (NRS) score between the first and last survey. a. Acute nausea. b. Delayed nausea.
The antiemetic guidelines provided by the American Society of Clinical Oncology (ASCO)2), National Comprehensive Cancer Network (NCCN)7), and the MASCC8) allowed us to control CINV using appropriate antiemetic prophylaxis. These antiemetic guidelines classify the risks of CINV based on the chemotherapy regimen (e.g., high emetic risk chemotherapy [HEC], moderate emetic risk chemotherapy [MEC], and low and minimal emetic risk chemotherapy). CINV is also classified based on the phase when it occurs (acute, delayed, and anticipatory nausea and vomiting).
The physiological mechanisms responsible for CINV continue to be elucidated and have provided a chance to develop antiemetic therapies1). The development of receptor antagonists targeting serotonin and neurokinin-1 has revolutionized the prevention of CINV, significantly reducing the incidence of CINV. Recently, new antiemetic agents, such as the 5-HT3RA, palonosetron, neurokinin-1 receptor antagonists, and aprepitant have been developed11). Palonosetron is a second-generation selective 5-HT3RA9). It has been shown to have an approximately 100-fold stronger binding affinity for the 5-HT3 receptor than that of first-generation 5-HT3RAs. It has an extended plasma elimination half-life of approximately 40 hr.
A combination of three antiemetics consisting of a 5-HT3RA, dexamethasone, and aprepitant is recommended for HEC, whereas a combination of a 5-HT3RA and dexamethasone is recommended for MEC11). Selective use of aprepitant and a second-generation 5-HT3RA is recommended; therefore, there are clinical issues concerning the selection of these drugs, especially MEC. In the present study, the clinical significance of the antiemetic protocol as evaluated using the MAT was demonstrated regarding the significant reduction in the NRS score for delayed nausea. The clinical use of the protocol may help in the realization that selective pharmacological prophylaxis for patients with CINV is possible, independent of the treating oncologist.
In this study, a combination of a first-generation 5-HT3RA (granisetron) and dexamethasone was administered to patients treated with oxaliplatin-based regimens, and a combination of a second-generation 5-HT3RA (palonosetron) and dexamethasone was administered to patients treated with irinotecan-based regimens; the latter had a stronger emetic effect than the oxaliplatin regimens10). We examined whether a first-generation 5-HT3RA was sufficient for the prophylaxis of an oxaliplatin-based regimen. In this study, there were no significant differences between the oxaliplatin- and irinotecan-based regimens, suggesting that palonosetron was not needed for the prevention of CINV in all patients treated with oxaliplatin-based regimens.
There was no significant reduction in acute emesis. This suggests that another prophylaxis for anticipatory CINV, such as olanzapine, would be needed in the revised protocol in the future.
In conclusion, the incidence of acute nausea was 30% and that of delayed nausea was 26% as evaluated using the MAT based on the guideline for recommended pharmacological prophylaxis. The clinical significance of the antiemetic protocol in the reduction of delayed nausea as evaluated using the MAT was also demonstrated. Further investigations are needed to clarify the efficacy of this protocol because this is a prospective study with a relatively small sample size.
We would like to express our gratitude to the staff of the ambulatory therapy center for their assistance in the collection and registration of data and samples.
Conflicts of interestManabu Shimomura and other co-authors have no conflicts of interest.