2015 Volume 84 Issue 2 Pages 161-171
2015 Volume 84 Issue 2 Pages 161-171
The effects of glycine betaine (GB) under heat stress conditions were studied in three marigold cultivars, namely, ‘Narai Yellow’, ‘Bali Gold’, and ‘Columbus Orange’. GB was foliarly applied to the seedlings 24 hours before transfer to either 25°C/25°C or 39°C/29°C (day/night) conditions for 15 days. Heat stress conditions caused photoinhibition and low levels of CO2 assimilation rate (A), stomatal conductance (gs), and transpiration rate (E) in all marigold cultivars compared with those of control plants grown under 25°C/25°C conditions. The accumulation of reactive oxygen species (ROS), lipid peroxidation, and cell death were also higher under heat stress in all cultivars compared with those in the control. However, the effect of heat stress on relative water content (RWC) was statistically significant only in ‘Bali Gold’. Foliar applications of GB at 0.5 and 1 mM alleviated photoinhibition and resulted in higher A, gs, and E in all marigold cultivars compared with those in the control under heat stress. Application of GB also resulted in lower levels of hydrogen peroxide, superoxide, lipid peroxidation, and cell death in all cultivars. The effect of GB on improving RWC was significant only in ‘Bali Gold’. In most cases, there were no significant differences between the effects of GB at 0.5 and 1 mM. The effect of GB seems to be very consistent across all marigold cultivars, as suggested by the lack of interaction between this effect and cultivars in most of the parameters that were studied. Overall, these results indicate that the foliar application of GB could possibly be used to mitigate the effect of heat stress in marigold.
Heat stress limits global crop production and is becoming more severe due to the current global warming trend. It has been estimated that an increase of temperature by one degree Celsius during the growing season could reduce the yields of corn and soybean by up to 17% (Lobell and Asner, 2003). Heat stress adversely affects crop production in many ways, such as by inhibiting seed germination, accelerating leaf senescence, reducing net photosynthesis and triggering early or delayed flowering (Wahid et al., 2007; Wheeler et al., 2000; Zinn et al., 2010). At the cellular level, heat stress may cause protein and membrane denaturation, damage of Photosystem II (PSII) and increased production of reactive oxygen species (ROS) (Suzuki and Mittler, 2006; Velikova and Loreto, 2005; Xu et al., 2006).
Glycine betaine (GB) is a quaternary ammonium compound that can be found in a wide range of bacterial, plant and animal species (Ashraf and Foolad, 2007; Chen and Murata, 2011; de Zwart et al., 2003). In some plants, accumulation of GB is a common response to alleviate the effect of abiotic stresses (Chen and Murata, 2011). Many studies have shown that increasing the cellular level of GB, either by a transgenic approach or by exogenous application, can effectively improve crop tolerance to various abiotic stresses including heat stress (Agboma et al., 1997; Alia et al., 1998; Makela et al., 1998; Rajashekar et al., 1999; Shahbaz et al., 2011; Xing and Rajashekar, 1999).
Although the exact mechanism is still unclear, it has been suggested that GB can mitigate heat stress via a number of different mechanisms. One of them is the protection of photosynthetic machinery (Chen and Murata, 2011). GB was found to stimulate synthesis of the D1 protein, which supports the repair of photodamaged PSII (Allakhverdiev et al., 2007). Furthermore, it has been shown that the maximum quantum efficiency of PSII photochemistry of a GB-deficient maize line declined much more than that of a GB-containing line when grown under heat stress conditions (Yang et al., 1996). The accumulation of GB prevented the sequestration of Rubisco activase to the thylakoid membrane, thereby maintaining the activity of Rubisco at a high temperature (Yang et al., 2005). Moreover, it has been found that the accumulation of GB alleviates the inhibition of net photosynthetic rate under heat stress (Wang et al., 2010).
The protective effect of GB under various stress conditions is also attributed to its capacity to increase the activity of some enzymes involved in the antioxidant defense system (Giri, 2011; Hoque et al., 2007). It has been reported that transgenic tobacco with the ability to synthesize GB had significantly lower levels of H2O2 and O2− than the wild type under heat stress conditions (Yang et al., 2007). This was possibly due to the increasing activity of superoxide dismutase and ascorbate peroxidase in the transgenic plants. In sugarcane, pre-soaking sprouting buds with GB was shown to inhibit H2O2 accumulation under heat stress (Rasheed et al., 2011). This previous study also indicated the significant negative relationship between H2O2 content and bud dry weight, suggesting that GB may improve thermotolerance mainly by limiting ROS generation. In transgenic wheat that had accumulated a high level of GB, it was found that the levels of several ROS in leaves of transgenic plants were lower than those of control plants under heat stress conditions (Wang et al., 2010).
The exogenous application of GB is a convenient method for the induction of crop tolerance to various abiotic stresses, but a possible disadvantage could be the lack of consistent results. The effect of GB seems to be strongly dependent on concentration, time and method of application, stress conditions, plant developmental stage, plant genotype, and species (Anjum et al., 2011; Ashraf and Foolad, 2007; Chen et al., 2009; Shahbaz et al., 2011; Sobahan et al., 2012; Zhang et al., 2009). In some cases, the application of GB has been shown to have no positive effect on crops under stress conditions (Ibrahim et al., 2006; Iqbal and Ashraf, 2006; Lin and Kao, 1995). These varied and inconsistent effects of GB hinder its utilization in crop production and emphasize the necessity of obtaining more information about the mechanism of GB-induced stress tolerance in different plant species and genotypes.
In the present study, the effect of exogenous GB application on heat stress was investigated in three different cultivars of marigold (Tagetes erecta), namely, ‘Narai Yellow’ (a tall and heat-tolerant cultivar bred for cut-flower production), ‘Bali Gold’ (an intermediate-height cultivar bred for cut-flower production), and ‘Columbus Orange’ (a dwarf cultivar bred for use as an ornamental pot and bedding plant). The major aims of this study are to determine whether GB can induce heat tolerance in marigold and to gain insight into the mechanism of GB-induced thermotolerance. Special emphasis is also placed on analysis of the interaction between the effect of GB and cultivars.
Marigold seeds of ‘Narai Yellow’, ‘Bali Gold’, and ‘Columbus Orange’ were obtained from AFM Flower Seed Co., Ltd., Chiang Mai, Thailand. These seeds were germinated and grown in pots placed in a tray. The growth medium consisted of peat moss, coarse sand and coconut coir (1:1:1, v/v). The growth conditions were 25°C/25°C (day/night), 16 hours of light per day and photosynthetic photon flux density of 200 μmol·m−2·s−1 (generated by light emission diodes 3000 K and 5000 K (1:1)). Plants were supplied with 0.5× Hoagland solution (Epstein and Bloom, 2005). At 20 days after sowing, seedlings of all cultivars were sprayed with glycine betaine (pharmaceutical grade; PureBulk, Inc., Roseburg, OR, USA) solution at 0.5 or 1 mM until run-off from all leaves (approximately 3 mL per seedling) for 24 hours before transfer to either 25°C/25°C or 39°C/29°C conditions for 15 days. The concentrations of glycine betaine were determined based on our preliminary results in which foliar application of glycine betaine at 1 mM resulted in the best improvement of heat tolerance, whereas higher concentrations did not have any positive effects and sometimes caused leaf scorch in marigold seedlings under heat stress conditions (data not shown). Heat stress conditions were determined according to our preliminary experiment in which a temperature higher than 39°C resulted in rapid wilting of marigold seedlings in less than 2 days. The control plants of 25°C/25°C and 39°C/29°C conditions were foliarly applied with deionized water. Throughout the experiment, water levels inside the trays for all treatments were kept constant by adding deionized water. All of the physiological analyses in this experiment were carried out after the marigold seedlings had been exposed to 25°C/25°C or 39°C/29°C conditions for 15 days.
Measurements of chlorophyll fluorescence parametersAll chlorophyll fluorescence parameters were measured using Fluorescence Monitoring System FMS2 (Hansatech Instruments Ltd., King’s Lynn, Norfolk, UK). Dark-adapted parameters were measured after all plants had been kept in the dark for 30 minutes. Maximum quantum efficiency of PSII photochemistry (Fv/Fm or (Fm–Fo)/Fm) and PSII operating efficiency (F'–Fm'/Fm' or ΦPSII) were calculated according to Baker (2008). The minimal fluorescence yield following dark adaptation, Fo, was determined by applying a modulation beam (< 0.05 μmol·m−2·s−1) and the average fluorescence value over 1.6 seconds was used as Fo. The maximal fluorescence following dark adaptation, Fm, was measured by applying a saturating light pulse (4000 μmol·m−2·s−1) and the highest average fluorescence from two consecutive data points was used as Fm. Steady-state fluorescence emission of light-adapted leaf, F', was determined by averaging fluorescence values under ambient light. Maximal fluorescence of light-adapted leaf, Fm', was determined similarly to Fm.
Measurement of gas exchange parameters and leaf surface temperatureAfter exposure to heat stress conditions for 15 days, leaf gas exchange parameters and leaf surface temperature were measured using LCi-SD (BioScientific Ltd., Hertfordshire, UK). Four of the youngest fully expanded leaves of 3 independent plants were measured for each cultivar and treatment. All of the measurements were carried out under photosynthetic photon flux density of 250 μmol·m−2·s−1.
In situ detection of hydrogen peroxide and superoxideHydrogen peroxide in leaf tissue was detected by using 3,3'-diaminobenzidine (DAB) solution following the protocol of Ahammed et al. (2012). Leaf discs were obtained from the youngest fully expanded leaves. The leaf discs were vacuum-infiltrated for 1 minute with 1 mg·mL−1 DAB solution at pH 3.8 (freshly prepared). Then, the leaf discs were incubated for 2 hours in the dark at room temperature. After incubation, chlorophyll was removed by boiling with 95% ethanol for 30 minutes. The hydrogen peroxide content in leaf tissues was represented by visible brown spots, which were the results of the reaction between hydrogen peroxide and DAB. These brownish areas and their intensity were quantified using the software ImageJ.
Superoxide in the leaf was detected by using nitroblue tetrazolium (NBT) following the protocol of Ramel et al. (2009). The leaf discs were vacuum-infiltrated with 0.5 mg·mL−1 NBT solution for 1 minute and incubated in the dark for 2 hours. Then, the leaf discs were boiled in a solution of acetic acid:glycerol:ethanol (1:1:3) for 20 minutes. The superoxide content in the leaf was represented by blue precipitation, which was the result of the reaction between NBT and superoxide.
Measurement of malondialdehyde (MDA)MDA content was measured by using a thiobarbituric acid-reactive substance (TBARS) assay as described by Heath and Packer (1968). Approximately 0.8 g of tissue was homogenized with 1 mL of 0.1% trichloroacetic acid (TCA) and centrifuged at 14000 g for 20 minutes. The supernatant was mixed with 0.5 mL of 0.5% thiobarbituric acid in 20% TCA (w/v) and incubated in water heated at 90°C for 25 minutes. Then, the samples were measured for absorbance at 440, 532, and 600 nm. The amount of MDA was calculated by using an extinction coefficient of 155 mM−1·cm−1. MDA content was determined by using the following equation:
Leaf cell death was detected by trypan blue staining following the protocol of Huang et al. (2011) with some modifications. The stock of trypan blue staining solution consisted of 10 g of phenol, 10 mL of glycerol, 10 mL of lactic acid, 10 mL of distilled water and 0.02 g of trypan blue. The leaf discs were incubated in the trypan blue working solution (stock solution:ethanol = 1:2, v/v) at 70°C for 1 minute and left at room temperature for 2 hours. Then, the leaf discs were incubated at 70°C for 20 minutes before taking pictures. The blue spots represent dead cells, which are unable to prevent the dye from entering them.
Measurement of relative water content (RWC)Leaf discs were weighed to determine fresh weight (FW). Then, they were submerged in distilled water overnight at room temperature to determine the turgid weight (TW). They were then dried at 85°C for 24 hours to determine the dry weight (DW). The relative water content was calculated as described by Yang and Lu (2006) using the following equation:
A complete randomized design was used for the experiment. All statistical analyses were performed by using SPSS V.22 (2013). Data are reported as mean ± standard error (SE). Significant differences among means were determined by one-way ANOVA with Duncan’s post hoc test at P < 0.05.
The effect of heat stress on PSII was evaluated by using chlorophyll fluorescence parameters. After 15 days of exposure to heat stress conditions (39°C/29°C), the PSII operating efficiency (ΦPSII) values of the marigold plants of all cultivars were significantly lower than those of plants grown under 25°C/25°C conditions. The value was found to be lowest in ‘Columbus Orange’. Foliar application of GB at 1 mM resulted in significantly higher values of ΦPSII in ‘Columbus Orange’ and ‘Bali Gold’ than those in the control under heat stress. The largest improvement was found in ‘Columbus Orange’. On the other hand, foliar application of GB had no significant effect on ΦPSII of ‘Narai Yellow’ (Fig. 1a).
Low values of maximum quantum efficiency of PSII photochemistry (Fv/Fm) were observed in all marigold cultivars grown under heat stress conditions, suggesting the occurrence of photoinhibition (Maxwell and Johnson, 2000). Similarly to the results of ΦPSII, ‘Columbus Orange’ exhibited the lowest Fv/Fm value (Fig. 1b). On the basis of the calculation method, the value of Fv/Fm can only decline by an increase of the Fo value, a decrease of the Fm value or both (Baker, 2008; Maxwell and Johnson, 2000). Therefore, Fo and Fm were examined to determine the cause of low Fv/Fm values. In ‘Bali Gold’ and ‘Columbus Orange’ grown under heat stress conditions, the Fo value was clearly higher than that of the control, whereas the Fm value was about the same or only slightly higher than that of the control. In contrast, the Fo value of ‘Narai Yellow’ was not significantly changed, whereas the Fm value was lower than that of the control under heat stress (Fig. 1c, d). Therefore, it appeared that the low values of Fv/Fm in ‘Bali Gold’ and ‘Columbus Orange’ were mainly due to the high value of Fo, whereas, in ‘Narai Yellow’, the low Fv/Fm value occurred due to the low value of Fm. Overall, foliar application of GB at both concentrations resulted in lower values of both Fo and Fm than those of the control under heat stress (Fig. 1c, d).

Effect of heat stress and GB on chlorophyll fluorescence parameters in three marigold cultivars. Foliar application of GB was performed 1 day before plants were transferred to heat stress conditions for 15 days. Control plants were sprayed with deionized water. (a) ΦPSII or operating efficiency of PSII in a light-adapted state. (b) Fv/Fm or maximum quantum efficiency of PSII in a dark-adapted state. (c) Fo or minimal fluorescence in a dark-adapted state. (d) Fm or maximal fluorescence in a dark-adapted state. Values are means ± SE (n = 4–6). Different letters indicate a significant difference (P < 0.05) between treatments only in the same cultivar. Significant difference was determined by using Duncan’s test.
In all marigold cultivars, exposure to heat stress for 15 days resulted in lower values of CO2 assimilation rate (A), stomatal conductance (gs), and transpiration rate (E) than those of plants grown under 25°C/25°C conditions (Fig. 2a, b, c). Foliar application of GB at both concentrations alleviated the effect of heat stress in all gas exchange parameters. The higher transpiration rate and stomatal conductance in GB-treated plants were associated with a lower leaf surface temperature (Fig. 2d).

Effect of heat stress and GB on (a) CO2 assimilation rate (A), (b) stomatal conductance (gs), (c) transpiration rate (E), and (d) leaf surface temperature. Values are means ± SE (n = 4). Different letters indicate a significant difference (P < 0.05) between treatments only in the same cultivar.
The amount of hydrogen peroxide (H2O2), a non-radical ROS, in marigold leaves was monitored by 3,3'-diaminobenzidine (DAB) staining. The results showed that the DAB-stained area, as represented by a dark-brown color, was more intense in leaf discs exposed to heat stress in all marigold cultivars (Fig. 3a, b). Foliar application of GB resulted in a smaller DAB-stained area with less intensity in all marigold cultivars. There was no significant difference in DAB-stained area between the applications of GB at 0.5 and 1 mM.

In situ detection of hydrogen peroxide by DAB staining in marigold leaf discs after exposure to heat stress conditions for 15 days. (a) Representative leaf discs stained with DAB of three marigold cultivars from the indicated treatments are shown. (b) DAB-stained area in leaf discs from each treatment. Color images of leaf discs were converted to black and white images and stained areas were analyzed using ImageJ. Values are means ± SE (n = 4). Different letters indicate a significant difference (P < 0.05) between treatments only in the same cultivar.
Superoxide contents were monitored by using nitroblue tetrazolium (NBT) staining. When compared with plants grown under 25°C/25°C conditions, the NBT-stained area was much more pronounced in leaves of all marigold cultivars that were exposed to heat stress (Fig. 4a, b). The application of GB at both concentrations resulted in a smaller NBT-stained area with less intensity. There was no significant difference in NBT-stained area between the application of GB at 0.5 and 1 mM in all marigold cultivars. Overall, these results suggest that the application of GB can greatly reduce the amounts of hydrogen peroxide and superoxide in marigold leaves compared with those in the control under heat stress conditions.

In situ detection of superoxide by NBT staining in marigold leaf discs after exposure to heat stress conditions for 15 days. (a) Representative leaf discs stained with NBT of three marigold cultivars from the indicated treatments are shown. (b) NBT-stained area in leaf discs from each treatment. Color images of leaf discs were converted to black and white images and stained areas were analyzed using ImageJ. Values are means ± SE (n = 4). Different letters indicate a significant difference (P < 0.05) between treatments only in the same cultivar.
The amount of MDA was higher in all marigold cultivars grown under heat stress conditions than in control plants grown at 25°C/25°C (Fig. 5). ‘Columbus Orange’ exhibited the largest response to heat stress as the MDA level of heat-stressed plants was higher than that of control plants by 125%. The application of GB resulted in a lower MDA level in all marigold cultivars, but the differences were statistically significant only in ‘Narai Yellow’. It was noteworthy that the application of GB at 0.5 mM resulted in a lower MDA level than GB at 1 mM in ‘Narai Yellow’.

Effect of heat stress on MDA content. Values are means ± SE (n = 4). Different letters indicate a significant difference (P < 0.05) between treatments only in the same cultivar.
The effect of heat stress on cell death was investigated by trypan blue staining. Cell death was found to be more prominent in leaves of plants exposed to heat stress, as seen by the larger area and higher intensity of trypan blue staining compared with those of control plants (Fig. 6a, b). Foliar application of GB at both concentrations resulted in less cell death in all marigold cultivars. Moreover, it was found that the application of GB at 0.5 mM resulted in less cell death than GB at 1 mM in ‘Narai Yellow’ and ‘Bali Gold’ under heat stress.
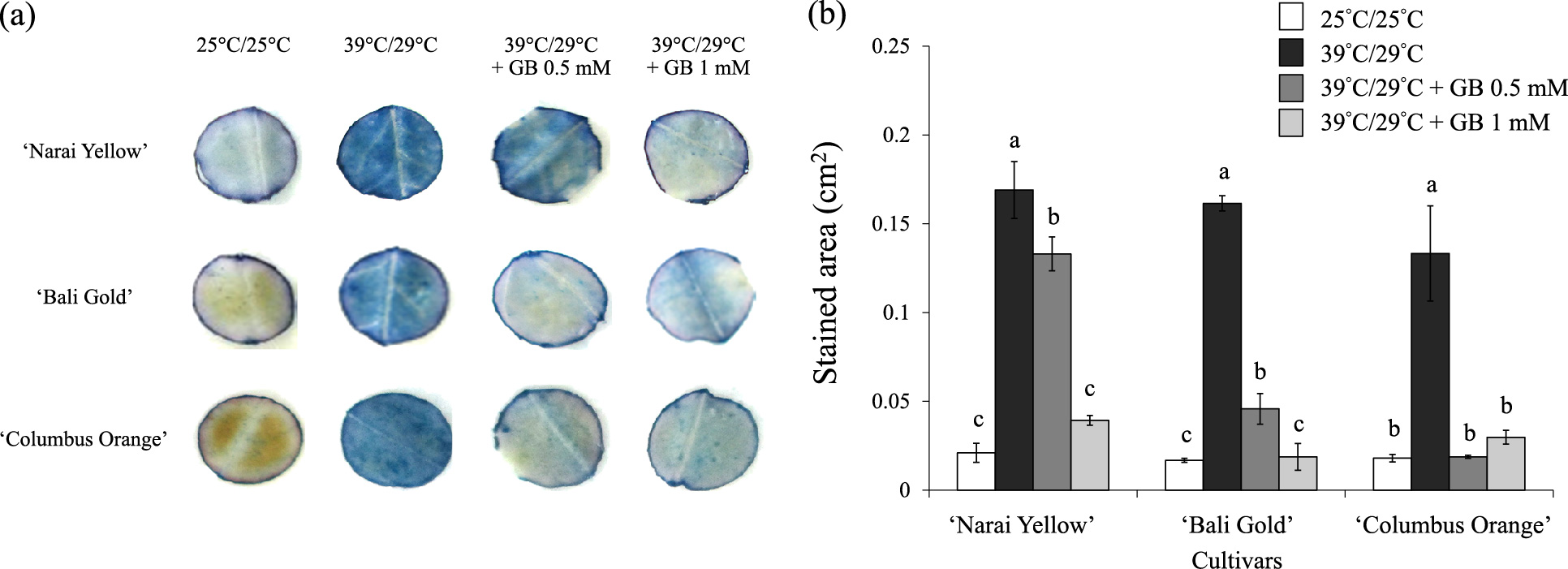
In situ detection of cell death by trypan blue staining in marigold leaf discs after exposure to heat stress conditions for 15 days. (a) Representative leaf discs stained with trypan blue of three marigold cultivars from the indicated treatments are shown. (b) Trypan blue-stained area in leaf discs from each treatment. Color images of leaf discs were converted to black and white images and stained areas were analyzed using ImageJ. Values are means ± SE (n = 4). Different letters indicate a significant difference (P < 0.05) between treatments only in the same cultivar.
Heat stress negatively affected RWC of all marigold cultivars, but the only cultivar that showed RWC that was significantly lower than that of the control was ‘Bali Gold’ (Fig. 7). The application of GB at both concentrations resulted in higher RWC in ‘Bali Gold’ than in the control under heat stress, whereas, in the other cultivars, GB did not have any significant effect on RWC.

Effect of heat stress on relative water content (RWC). Values are means ± SE (n = 4). Different letters indicate a significant difference (P < 0.05) between treatments only in the same cultivar.
To understand the effect of GB on the physiological characteristics of marigold, the parameters of marigold cultivars and their interaction on marigold under heat stress conditions, two-way ANOVA was performed. It was found that the effect of foliar application of GB was significant in all parameters except for RWC (Table 1). The effect of cultivars was significant in almost every parameter except for A, H2O2, and RWC. In contrast, the interaction between GB and cultivars was significant only in 4 of the 13 parameters studied.

The significance levels, calculated by two-way ANOVA, of the effects of cultivar (CV), GB, and their interaction (CV × GB) on all parameters under heat stress conditions are shown as p-values. The levels of hydrogen peroxide (H2O2), superoxide (O2• −), and cell death were quantified from DAB-, NBT-, and trypan blue-stained areas on leaf discs, respectively.
The exogenous application of GB is a safe and simple method that has been successfully used to improve heat stress tolerance in tomato (Li et al., 2011; Makela et al., 1998) and sugarcane (Rasheed et al., 2011). In this study, the results show that foliar application of GB can effectively mitigate the effect of heat stress in 3 marigold cultivars. The GB-treated marigold plants exhibited less inhibition of photosynthesis, lower leaf surface temperature and lower levels of ROS and cell death under heat stress conditions.
In marigold, the impact of heat stress on PSII is evident. The low values of ΦPSII and Fv/Fm under heat stress conditions indicate that the efficiency of PSII has deteriorated (Fig. 1a, b), which may partially explain the low net photosynthesis rate (Fig. 2a). This is consistent with previous reports showing that ΦPSII has a strong relationship with the net photosynthesis rate (Fryer et al., 1998; Yin et al., 2010). Furthermore, the results show that the low values of Fv/Fm under heat stress in ‘Bali Gold’ and ‘Columbus Orange’ are mainly due to the high values of Fo, whereas in ‘Narai Yellow’, it is due to the low value of Fm (Fig. 1c, d). A high value of Fo was found to correlate with damage to chloroplasts and PSII (Demmig and Björkman, 1987; Yamane et al., 2008). Thus, the damage to PSII and the photosynthetic apparatus in ‘Narai Yellow’ under heat stress is possibly less severe than in the other two cultivars.
Foliar application of GB resulted in higher values of ΦPSII and Fv/Fm in all marigold cultivars under heat stress conditions than in the control (Fig. 1a, b). For Fv/Fm, these higher values occurred due to the lower values of both Fo and Fm. A low value of Fo may occur because of the effect of GB on stimulating the repair of photodamaged PSII (Allakhverdiev et al., 2007). On the other hand, it has been suggested that a decrease of Fo in direct proportion to Fm may occur because of the activity of non-photochemical quenching (NPQ) processes, such as qE (energy-dependent quenching), which involve the conversion of excess light energy into heat in order to avoid damage to the photosynthetic system (Baker, 2008; Gilmore et al., 1996; Müller et al., 2001). Hence, it is tempting to hypothesize that GB may also protect PSII by stimulating NPQ processes. However, some researchers have noted that the accumulation of GB, either by a transgenic approach or by exogenous application, causes reduction of NPQ in tobacco and maize under salt stress conditions (Yang and Lu, 2005; Yang et al., 2008). Obviously, this contradiction may occur because of differences in the level of GB accumulation, type of stress and plant species. Further experiments should be performed to compare the effect of GB on NPQ processes under different stresses and plant species.
All of the gas exchange parameters exhibited very similar patterns in this experiment. Exposure to heat stress resulted in lower values of A, gs, and E than in control plants grown under 25°C/25°C conditions, whereas the application of GB resulted in higher values (Fig. 2a, b, c). GB treatment was found to increase gs under stress and normal conditions (Makela et al., 1998; Yang and Lu, 2006). Thus, the higher values of A and E in this experiment could be attributed to the effect of GB on gs. Furthermore, the higher E and gs values in GB-treated plants may also support heat dissipation from the leaves, resulting in a lower leaf surface temperature, as seen in Figure 2d. This slightly but significantly lower leaf surface temperature might indirectly contribute to the mitigating effect of GB under heat stress conditions.
Heat stress is known to induce the over-production of ROS, which can cause severe damage to cellular components and eventually cell death (Breusegem and Dat, 2006; Suzuki and Mittler, 2006; Wahid et al., 2007; Xu et al., 2006). As shown in Figures 3 and 4, heat stress caused high accumulation of hydrogen peroxide and superoxide in all marigold cultivars. Accumulations of these ROS might have contributed to the high levels of cell death, as shown in Figure 6. Foliar application of GB under heat stress conditions resulted in much lower levels of hydrogen peroxide, superoxide and cell death in leaf tissue of all marigold cultivars. Our results are consistent with previous studies showing that the accumulation of GB in transgenic tobacco and wheat can indirectly decrease the levels of hydrogen peroxide and superoxide under heat stress conditions by increasing the activity of some antioxidant enzymes, such as superoxide dismutase, catalase, and ascorbate peroxidase (Wang et al., 2010; Yang et al., 2007). To investigate further the effect of GB on ROS under heat stress, the level of lipid peroxidation was evaluated by measuring the amount of MDA, a highly toxic by-product of lipid peroxidation (Hodges et al., 1999). The results showed that the application of GB resulted in moderately less MDA in all marigold cultivars under heat stress compared with that in the control (Fig. 5). This slight disagreement between the effect of GB on hydrogen peroxide and superoxide contents and MDA contents could be explained by the fact that lipid peroxidation is generally caused by singlet oxygen and hydroxyl radicals, and rarely by hydrogen peroxide and superoxide (Møller et al., 2007). Thus, it is likely that the ROS scavenging mechanism of GB varies among different types of ROS.
There are some reports indicating that the accumulation of GB can improve plant water status under stress conditions (Anjum et al., 2012; Lv et al., 2007; Xing and Rajashekar, 1999). The effects of heat stress and GB on RWC of marigold were unclear in our experiment. The results revealed that heat stress resulted in lower RWC than in the control and the application of GB can significantly improve RWC only in ‘Bali Gold’ (Fig. 7). For the other two cultivars, the effects of heat stress and GB on RWC were not statistically significant. These results are consistent with a study on grapevine in which the exogenous application of GB did not affect RWC (Mickelbart et al., 2006). Similarly, a previous study by Wang et al. (2010) also indicated that heat stress and the accumulation of GB do not affect RWC in transgenic wheat. Nevertheless, the authors also show that GB may indirectly improve osmotic adjustment during heat stress.
It is likely that the three marigold cultivars used in this study differed in terms of heat tolerance, as seen in the ANOVA results (Table 1). On the basis of the comparison of all physiological parameters under heat stress conditions, ‘Narai Yellow’ and ‘Bali Gold’ tend to exhibit greater heat tolerance than ‘Columbus Orange’. The effect of GB seems to be very consistent in all marigold cultivars, as seen by the lack of interaction between the effect of GB and cultivars in most of the parameters that have been studied (Table 1). Our results are in accordance with studies on maize in which the effect of GB was consistent among different maize cultivars under salt stress conditions (Kausar et al., 2014; Nawaz and Ashraf, 2007). In contrast, there are also some reports showing that the effect of GB appeared to vary among cultivars. For example, it was found that the interactions between the effect of GB and wheat cultivars were significant in 13 of the 20 investigated parameters (Shahbaz et al., 2011). In another study, exogenous application of GB under salt stress conditions significantly increased the fresh weight and potassium uptake of rice ‘Nipponbare’, but not those of rice ‘Pokkali’ (Sobahan et al., 2012). The variation of cultivar response to the application of GB in a plant species could be explained by the differences in genetic background between the cultivars used in the experiment. In our case, all marigold cultivars originated from the same company and their phenotypes were quite similar in many aspects, except heat tolerance and height. It is possible that these marigold cultivars share very similar genetic backgrounds and therefore respond to the application of GB in similar ways.
In conclusion, ‘Narai Yellow’ and ‘Bali Gold’ seem to exhibit greater heat tolerance than ‘Columbus Orange’. The application of GB at 0.5 and 1 mM can similarly and effectively mitigate the negative effect of heat stress in all marigold cultivars. The mechanism of this mitigation might involve the protection of photosynthetic machinery, increasing gs and ROS scavenging. The role of GB in improving water status was unclear in our experiment. These results suggest the possibility of using the foliar application of GB to alleviate the effect of heat stress in marigold under field conditions.
This work was partially supported by the Faculty of Agricultural Production, Maejo University, Chiang Mai. We wish to thank Mr. Clive Richardson and AFM Flower Seed (Thailand) Co., Ltd. for supporting us by providing seeds and some important information about growing marigold throughout the experiment. We also thank the Institute of Product Quality and Standardization (IQS) of Maejo University for supporting us by providing access to the photosynthesis system and some other lab equipment. Finally, we thank Dr. Preeda Nathewet for reviewing our manuscript.