2016 Volume 85 Issue 1 Pages 76-85
2016 Volume 85 Issue 1 Pages 76-85
In order to extend the “eating window”, the optimum ripening phase suitable for eating, the combination of treatment with propylene (an ethylene analog) and 1-methylcyclopropene (1-MCP; an ethylene inhibitor) was assessed in three kiwifruit cultivars: ‘Rainbow Red’ Actinidia chinensis, ‘Sanuki Gold’ A. chinensis, and ‘Hayward’ A. deliciosa. Propylene treatment initiated the ripening process by inducing fruit softening, increasing soluble solid content (SSC), and decreasing titratable acids (TA), with or without endogenous ethylene production, depending on the duration of exposure. Once endogenous ethylene was induced, it accelerated fruit ripening, resulting in an over-ripening phase and shortening of the “eating window”. ‘Rainbow Red’ and ‘Sanuki Gold’ fruit treated with propylene continuously or for 48 h initiated endogenous ethylene production that led to an “eating window” lasting only 2 days (range of 3–5 days after the start of treatment), whereas it lasted for 7 days (range 3–10 days) in ‘Hayward’ fruit. Limited propylene treatment of the three cultivars for 24 h induced ripening without the detection of ethylene production, suggesting that the optimum ripening phase suitable for eating can be attained without endogenous ethylene production, resulting in a longer “eating window”. ‘Rainbow Red’ and ‘Sanuki Gold’ fruit treated with propylene for 48 h followed by 1-MCP treatment had extended “eating window” and shelf-life, with the suppression of endogenous ethylene. These results illustrate the practicability of different durations of propylene treatment in facilitating kiwifruit ripening and the additional benefit of 1-MCP treatment to the extend shelf-life of new high-quality kiwifruit cultivars, ‘Rainbow Red’ and ‘Sanuki Gold’.
Kiwifruit belongs to the genus Actinidia which is composed of more than 76 species having wide diversity in phenotype, flavor, color, acidity level, and soluble sugar content (Ferguson and Huang, 2007; Nishiyama, 2007; Nishiyama et al., 2008). Kiwifruit is a climacteric fleshy fruit sensitive to postharvest exogenous ethylene treatment that induces physical and physiological changes, thus stimulating the ripening process (Crisosto et al., 1997; Mworia et al., 2010; Park et al., 2006; Sfakiotakis et al., 1997). The leading cultivar of kiwifruit on the international market is Actinidia deliciosa ‘Hayward’, which is a hardy cultivar with relatively low soluble solid content (SSC) of 11%–14% and titratable acid (TA) of 1.5%–0.8% when ripe. ‘Hayward’ kiwifruit is a late harvesting cultivar that is considered to be among the first generation of cultivated kiwifruit. In recent years, there has been increasing demand for more improved and diverse kiwifruit cultivars, prompting the release of new high-quality cultivars such as A. chinensis ‘Hort16A’ with yellow flesh, low TA, and high SSC of ~16%. In Japan, two new kiwifruit cultivars, ‘Sanuki Gold’ and ‘Rainbow Red’, belonging to A. chinensis have gained a good reputation in recent years. These two are early harvesting cultivars (about 1 month before ‘Hayward’ kiwifruit) and are considered as the second generation of kiwifruit with premium quality, associated with relatively high SSC and low TA (Fukuda et al., 2007; Murakami et al., 2014). ‘Sanuki Gold’ kiwifruit is a tetraploid cultivar characterized by large fruit size, golden yellow flesh color, and brown fruit skin, whereas ‘Rainbow Red’ kiwifruit is small in size (100 g), has green skin, yellow flesh, and reddish coloration within the inner pericarp. The best eating quality for ‘Sanuki Gold’ and ‘Rainbow Red’ kiwifruit is achieved when fruit have an SSC level higher than 16% and TA of around 1%. However, low TA of less than 0.8% makes the fruit too sweet, due to an inadequate balance between SSC and TA, thus affecting taste and consequently affecting consumers’ acceptance. ‘Hayward’ kiwifruit with high SSC (14%) and low TA are most readily accepted by consumers (Crisosto and Crisosto, 2001). Previous research has shown that, in ‘Hayward’ and ‘Hort16A’ kiwifruit, fruit firmness decreased after ethylene treatment (Crisosto et al., 1997; Richardson et al., 2011; Ritenour et al., 1999). Currently, most of the postharvest protocols for kiwifruit ripening are based on the results obtained from ‘Hayward’ and ‘Hort16A’ kiwifruit. The introduction of new cultivars, however, necessitates an understanding of the postharvest physiological responses in individual kiwifruit cultivars.
The application of exogenous ethylene or its analogue propylene accelerates the ripening of kiwifruit in four main phases, as hypothesized by Atkinson et al. (2011) and Schröder and Atkinson (2006), and as shown in Figure 6. Phase I is the pre-climacteric (unripe) stage characterized by high firmness, high TA, and low SSC, making kiwifruit unsuitable for consumption. Phase II is characterized by the greatest loss of fruit firmness without the detection of endogenous ethylene production, while in Phase III, kiwifruit enter the “eating window” with high SSC, low TA, and autocatalytic ethylene production. Phase IV is the overripe stage, when fruit are not recommended for consumption (see Fig. 6). Crisosto et al. (1997) proposed preconditioning before shipment with the combination of ethylene and storage/transit temperature to induce ripening in ‘Hayward’ kiwifruit. Kiwifruit exhibit an asynchronous pattern between softening and climacteric rise in ethylene, which provides a modality for extending the “eating window” of fruit during ripening. In transgenic kiwifruit lines with ACC oxidase knocked down and no detectable levels of ethylene, even after ethylene treatment of 100 μL·L−1 for 24 h, Phase III fruit softening was arrested, thereby extending the edible firmness of fruit by > 25 days (Atkinson et al., 2011). The use of propylene to trigger ethylene biosynthesis in fruit is beneficial for distinguishing exogenous ethylene from endogenous ethylene produced by fruit themselves (McMurchie et al., 1972). Thus, determining the duration of exposure to propylene required to induce ripening in different kiwifruit cultivars may extend our understanding of the implications of ethylene for kiwifruit physiological properties and practical postharvest application. This would be beneficial in deducing the optimum “edible window” of kiwifruit cultivars during ripening before the onset of the overripe stage at Phase IV.
1-Methylcyclopropene (1-MCP), a potent inhibitor of ethylene perception, has revolutionized the fruit industry by delaying ripening, thus extending postharvest life in climacteric fruit (Sisler et al., 1996; Watkins, 2006). ‘Hayward’ kiwifruit treated with 1-MCP before cold storage had delayed ripening even after fruit were transferred to 20°C (Boquete et al., 2004). Moreover, the application of 1-MCP after ethylene treatment has also been shown to suppress ethylene biosynthesis and softening in ‘Sanuki Gold’ kiwifruit (Mworia et al., 2012), ‘Anna’ apple (Pre-Aymard et al., 2003), ‘Charentais’ melon (Nishiyama et al., 2007), and ‘La France’ pear (Kubo et al., 2003). Most research on 1-MCP so far has concentrated on application before the long-term storage of kiwifruit. However, information on the use of 1-MCP during short-term shelf-life storage and ripening is limited. Such information could be beneficial in assessing the impact of 1-MCP treatment after ethylene/propylene treatment in extending the shelf-life of kiwifruit. This will ensure kiwifruit are available to consumers when they are still at their premium edible stage (Ritenour et al., 1999).
The short postharvest life of kiwifruit cultivars is largely attributed to rapid softening after ethylene treatment (Terasaki et al., 2013). Another limiting factor in the postharvest storage of kiwifruit is the incidence of fruit rot caused by fungi at temperatures above 20°C (Kinugawa, 2000; Koh et al., 2005; Yano and Hasegawa, 1993). Koh et al. (2005) attributed the prevalence of ‘Hayward’ kiwifruit rot to fungi such as Phomopsis sp., Botryosphaeria sp., and Diaporthe sp. and deduced that, for kiwifruit stored at 17°C–29°C, about 20% to 95% of fruit developed rot symptoms after 20 days of storage. In order to reduce fruit rot incidence, lower temperatures such as 10°C or 15°C are recommended during postharvest fruit ripening.
Variation among different kiwifruit cultivars necessitates determination of the optimum ripening conditions for individual cultivars in order to obtain premium fruit with a longer shelf-life. Harmonization of ripening characteristics such as fruit firmness, SSC, and TA levels is important to consumers in order to harness nutritional advantages and prolonged shelf-life (Bapat et al., 2010; Bruhn, 1995; Crisosto and Crisosto, 2001; Semmelmeyer, 2006; Valero et al., 2007). Our overall goal is to assess ways of extending the optimum “eating window” of kiwifruit considering cultivar differences. Therefore, the objective of this study was to assess the responses of three kiwifruit cultivars, ‘Rainbow Red’, ‘Sanuki Gold’, and ‘Hayward’, to different durations of exposure to propylene in stimulating ripening. 1-MCP application after propylene treatment was used to extend the postharvest shelf-life.
Actinidia chinensis ‘Rainbow Red’, A. chinensis ‘Sanuki Gold’, and A. deliciosa ‘Hayward’ kiwifruit were used in this study. ‘Rainbow Red’ kiwifruit was obtained from Sakaide City, ‘Sanuki Gold’ from Takamatsu City, and ‘Hayward’ fruit from the experimental orchard at Okayama University. The experiment was conducted twice in 2010 and 2011 harvesting seasons achieving similar results; hence, the results for the 2011 season are presented in this study. The harvesting dates were based on commercial harvesting times for each cultivar, which were 25 September for ‘Rainbow Red’, 7 October for ‘Sanuki Gold’, and 10 November for ‘Hayward’. After harvesting, fruit were immediately transferred to Okayama University where they were sorted to uniform fruit size and confirmed to be free from defects and blemishes.
Propylene and 1-MCP treatmentsFruit of each kiwifruit cultivar were divided into 8 groups. Group 1–4 fruit were treated with 5000 μL·L−1 propylene (McMurchie et al., 1972) for 12 h, 24 h, 48 h, or continuously. Group 5–7 fruit were treated with propylene for 12 h, 24 h, or 48 h, followed by 1-MCP treatment at a concentration of 5 μL·L−1 for 12 h (SmartFreshSM powder, active ingredient 0.14%; Rohm and Hass, Philadelphia, PA, USA) according to the protocol of Mworia et al. (2012). Group 8 consisted of untreated control fruit. Thus, 8 groups were established for each of the three kiwifruit cultivars, namely, untreated control (Control), propylene 12 h (P12h), propylene 24 h (P24h), propylene 48 h (P48h), propylene 12 h + 1-MCP (P12hMCP), propylene 24 h + 1-MCP (P24hMCP), propylene 48 h + 1-MCP (P48hMCP), and propylene continuous (P-cont). Each group was comprised of 20–30 fruit. All fruit were then stored in polylined trays at 15°C during the ripening period.
Evaluation of ethylene, fruit firmness, SSC, and TAEthylene production, flesh firmness, SSC, and TA of fruit were analyzed at harvest (day 0), day 1, day 2, day 3, day 5, day 7, and day 10. Each sampling point consisted of five fruit. Ethylene measurement was conducted using a gas chromatograph (model GC-8A; Shimadzu, Kyoto, Japan), equipped with a flame ionization detector (set at 200°C) and an activated alumina column (set at 80°C), as previously described by Mworia et al. (2010). Fruit skin from opposite sides of a fruit at the central part was removed and then outer pericarp firmness (Fig. 1) was measured using a penetrometer (model SMT-T-50; Toyo Baldwin, Tokyo, Japan) fitted with a 5 mm plunger at a speed of 30 mm·min−1; then, fruit were cut in half and core firmness was determined. The firmness reading obtained is expressed in Newtons (N). The other half of fruit was peeled and homogenized fruit pieces were squeezed through cheesecloth to extract fruit juice. SSC was measured using a digital refractometer (Atago Co. Ltd., Tokyo, Japan) and is expressed as Brix (%). TA was determined by diluting the fruit juice with distilled water at a ratio of 1:5; then, the solution was titrated against 0.1 N NaOH using phenolphthalein to indicate the end-point of acid neutralization. TA is expressed as percentage citric acid equivalents (%TA).
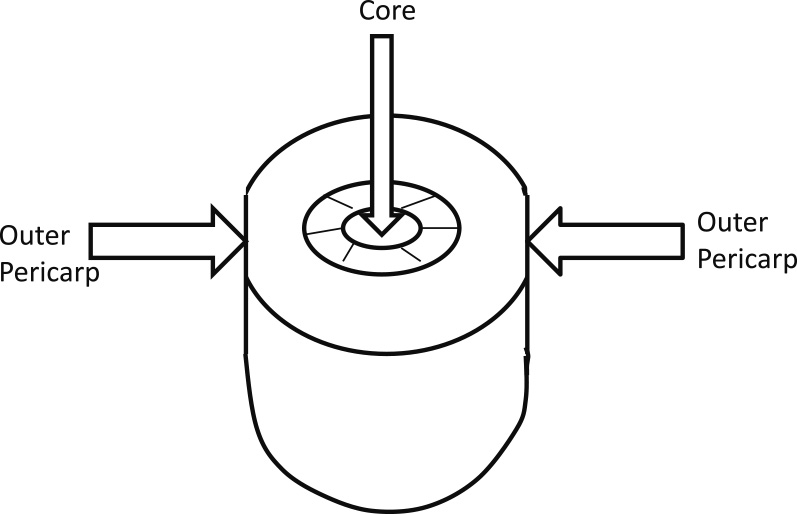
Schematic diagram of firmness measurement of outer pericarp and core in kiwifruit.
Ethylene production was detected in fruit treated with propylene continuously on day 5 for ‘Rainbow Red’, day 3 for ‘Sanuki Gold’, and day 7 for ‘Hayward’ fruit and then ethylene production increased as ripening progressed (Fig. 2). ‘Rainbow Red’ and ‘Sanuki Gold’ fruit treated with propylene for 48 h initiated ethylene biosynthesis by day 5. Conversely, in ‘Hayward’ kiwifruit, ethylene production was not detected for fruit treated with propylene for 48 h during the 10 days of the study. 1-MCP treatment was effective at suppressing ethylene production for ‘Rainbow Red’ fruit treated with propylene for 48 h. Exposure of the fruit to 1-MCP after 24 h and 48 h of propylene treatment similarly inhibited ethylene production in ‘Sanuki Gold’ fruit. In all cultivars, fruit treated with propylene for 12 h with or without 1-MCP treatment and control fruit did not produce detectable levels of ethylene during 10 days of storage.
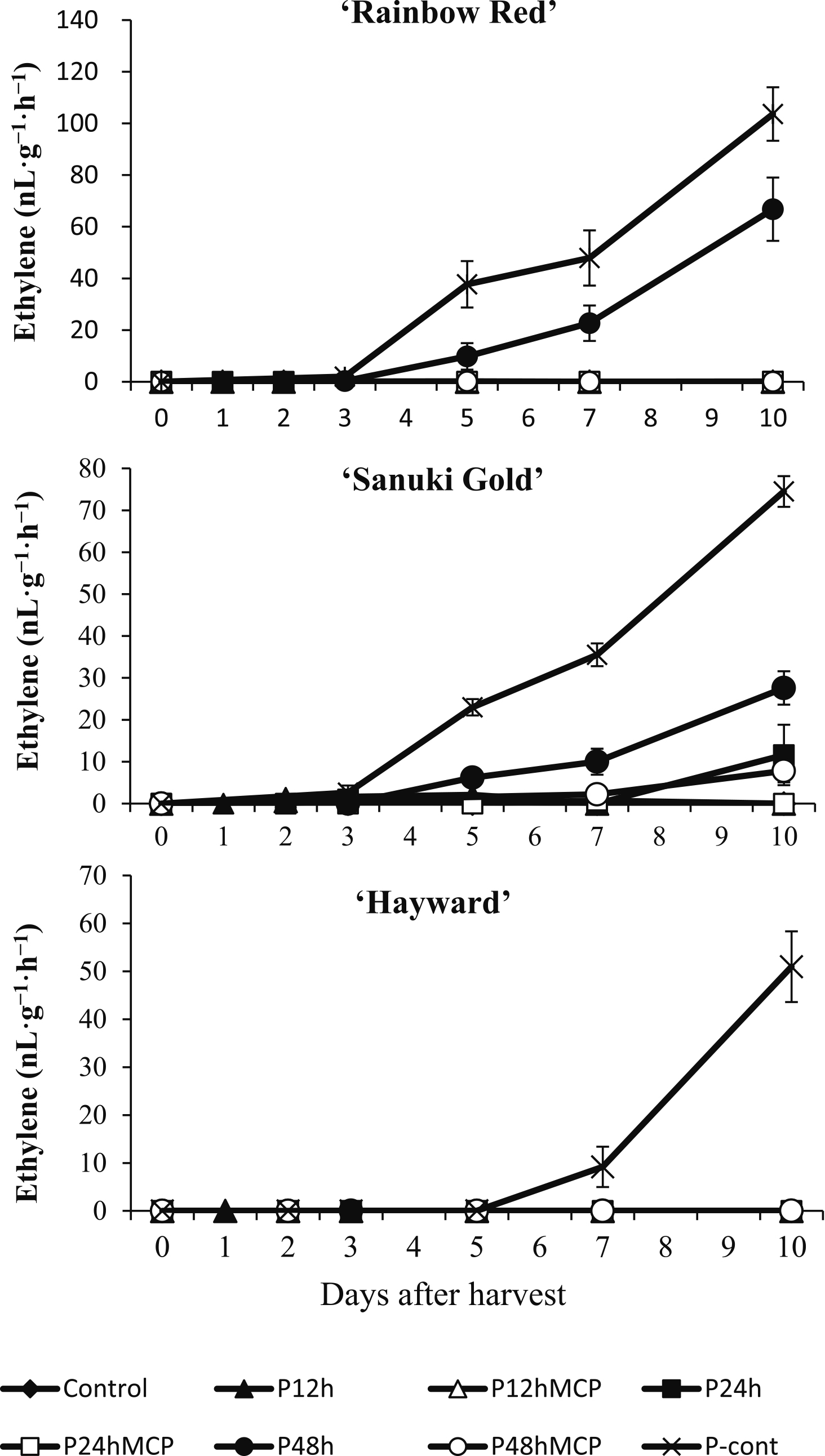
Effect of propylene and 1-MCP treatments on ethylene production of kiwifruit cultivars ‘Rainbow Red’, ‘Sanuki Gold’, and ‘Hayward’. Each data point is from measurements of 5 fruit with SE bars.
Figure 3 depicts the core and outer pericarp firmness of three kiwifruit cultivars and optimum eating firmness range, as indicated by the gray region. The upper and lower limits of the eating range were based on a panel test by assessors in order to determine the optimum firmness accepted by consumers. This showed that kiwifruit edible eating firmness had ranges of 5–25 N and 4–10 N for core and outer pericarp, respectively (Fig. 3). ‘Rainbow Red’ fruit treated with propylene continuously had drastic decreases in core and outer pericarp firmness within 3 days to attain the “eating window” and had surpassed the “eating window” by day 5, suggesting that the fruit had reached the overripe stage. Fruit treated with propylene for 48 h decreased in firmness within 3 days to achieve eating firmness of < 10 N for both core and outer pericarp. The “eating window” for ‘Rainbow Red’ fruit treated with propylene for 48 h was between 3 and 5 days. The exposure of fruit to 1-MCP after 48 h of propylene treatment further delayed core softening in ‘Rainbow Red’ fruit, hence extended the “eating window” to 10 days. The core of ‘Rainbow Red’ fruit treated with propylene for 24 h with or without 1-MCP treatment gradually softened, reaching eating quality by day 7, although the outer pericarp reached the eating level earlier, on day 5. Propylene treatment for 12 h with or without 1-MCP treatment was insufficient to accelerate ‘Rainbow Red’ fruit softening for both core and outer pericarp. Core and outer pericarp of untreated control fruit were firm and did not attain suitable softness for eating during the 10 days.
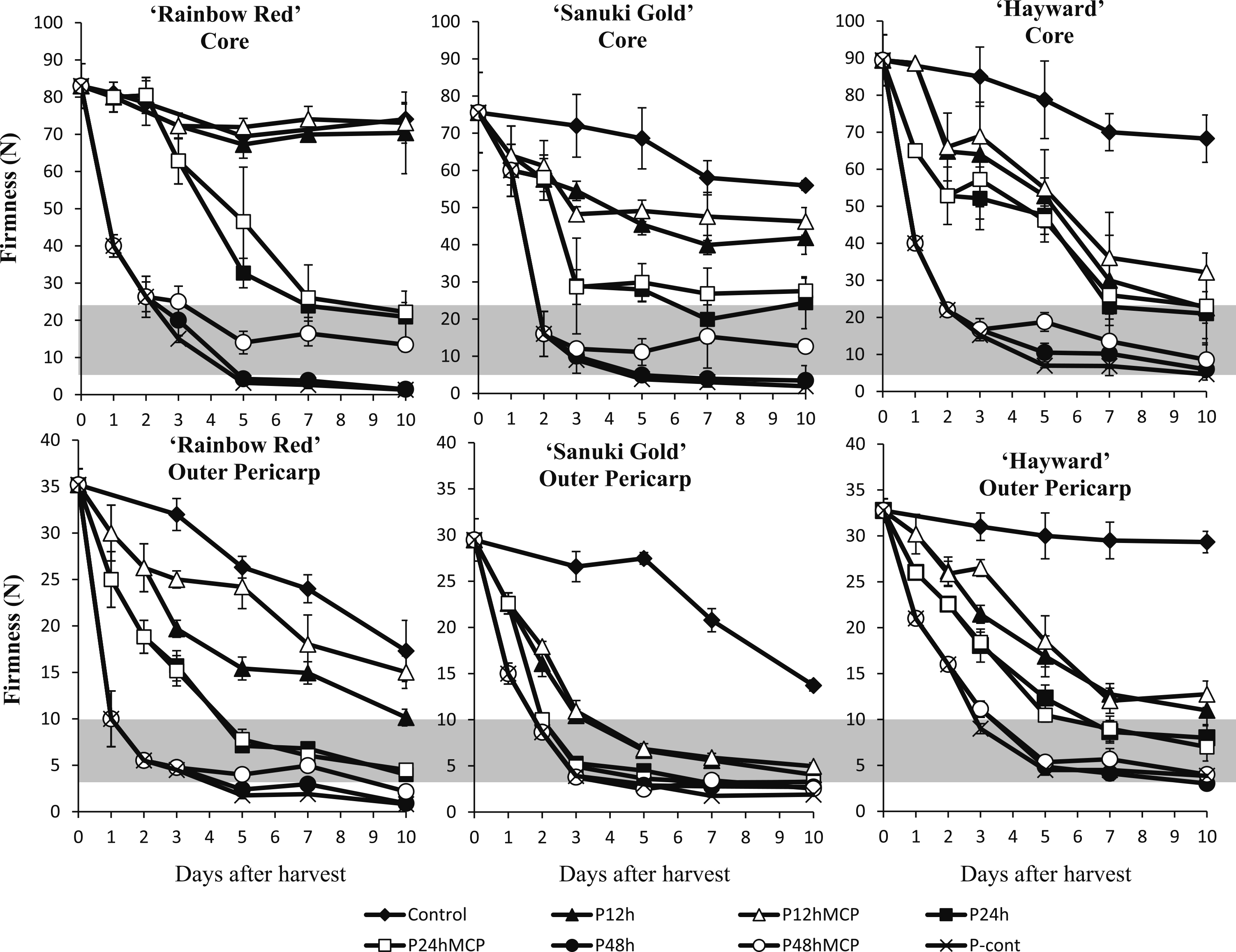
Effect of propylene and 1-MCP treatments on core and outer pericarp firmness of kiwifruit cultivars ‘Rainbow Red’, ‘Sanuki Gold’, and ‘Hayward’. Gray region indicates the optimum eating stage. Each data point is from measurements of 5 fruit with SE bars.
The core firmness of ‘Sanuki Gold’ fruit treated with propylene continuously or for 48 h with or without 1-MCP treatment decreased drastically to reach a level suitable for eating by day 3. The core firmness of ‘Sanuki Gold’ fruit treated with propylene for 48 h was within the “eating window” for 3 to 5 days, whereas 1-MCP treatment after 48 h of propylene treatment extended the optimum eating period for longer than 10 days. The core of ‘Sanuki Gold’ fruit treated with propylene for 24 h attained suitable firmness for eating on day 7, but fruit treated with propylene for 24 h followed by 1-MCP treatment did not attain eating firmness. The outer pericarp of ‘Sanuki Gold’ fruit gradually softened for all durations of propylene treatment to attain suitable firmness for eating from day 3, but not in control fruit.
The core of ‘Hayward’ fruit treated with propylene continuously or for 48 h with or without 1-MCP treatment softened to 18 N on day 3 and remained in the “eating window” up to day 10. ‘Hayward’ fruit treated with propylene for 24 h with or without 1-MCP treatment softened gradually, attaining suitable firmness for eating on day 7. The outer pericarp of ‘Hayward’ fruit treated with propylene for 24 h reached acceptable firmness for eating on the same days as the fruit core. ‘Hayward’ fruit treated with propylene for 12 h with or without 1-MCP treatment and control fruit did not attain suitable firmness for eating for both core and outer pericarp within the 10 days of the study.
Soluble solid content and titratable acids of kiwifruit during ripeningThe recommended level of SSC for eating was set at > 15% for ‘Rainbow Red’ and ‘Sanuki Gold’ fruit and > 12% for ‘Hayward’ fruit in this experiment (Fig. 4). ‘Rainbow Red’ and ‘Sanuki Gold’ fruit treated with propylene continuously or for 48 h with or without 1-MCP treatment attained an acceptable SSC level of > 15% from day 5 and maintained this high SSC level until the end of the study. ‘Hayward’ fruit treated with propylene continuously and for 48 h with or without 1-MCP treatment surpassed the recommended SSC level of > 12% on day 5. ‘Rainbow Red’ and ‘Sanuki Gold’ fruit treated with propylene for 24 h had a delayed increase in SSC, reaching acceptable SSC levels on day 7. ‘Hayward’ fruit treated with propylene for 24 h with or without 1-MCP treatment almost reached edible levels of SSC on day 10. Propylene treatment for 12 h with or without 1-MCP treatment did not induce increases in SSC to acceptable levels in ‘Rainbow Red’ and ‘Hayward’ fruit, but in ‘Sanuki Gold’ without 1-MCP, fruit attained an edible SSC level on day 10. In all of the three kiwifruit cultivars, control fruit did not achieve an SSC level suitable for consumption.
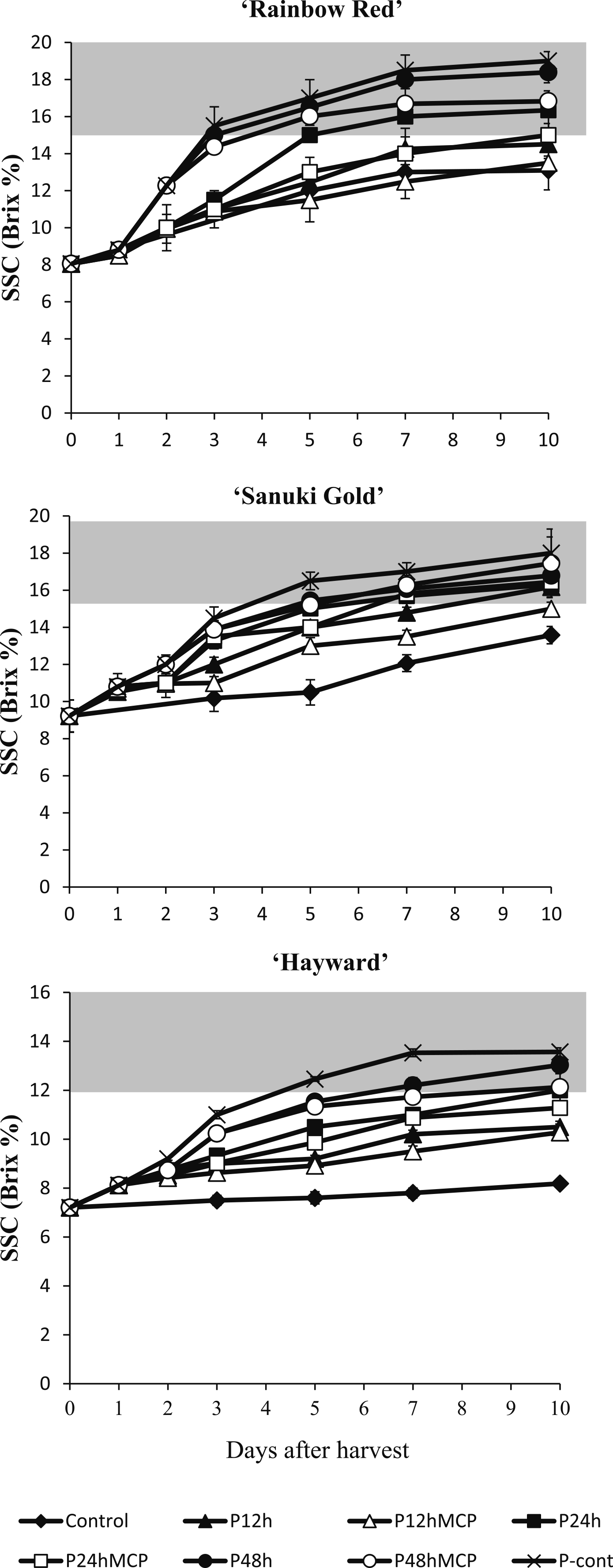
Effect of propylene and 1-MCP treatments on soluble solid content (SSC) of kiwifruit cultivars ‘Rainbow Red’, ‘Sanuki Gold’, and ‘Hayward’. Gray region indicates the optimum eating stage. Each data point is from measurements of 5 fruit with SE bars.
In this experiment, the recommended level of TA for eating was set at in the range of 0.8%–1.2%. Fruit treated with propylene continuously or for 48 h had a drastic reduction in TA in all of the kiwifruit cultivars. ‘Rainbow Red’ and ‘Sanuki Gold’ fruit treated continuously with propylene showed decreases in TA, reaching levels below 0.8% by day 3 and day 7, respectively (Fig. 5). 1-MCP treatment after 48 h of propylene treatment extended the optimum “eating window” of TA in ‘Rainbow Red’ and ‘Sanuki Gold’ fruit up to 10 days. ‘Rainbow Red’ and ‘Sanuki Gold’ fruit treated with propylene for 24 h with or without 1-MCP treatment reached the recommended TA from day 5. In ‘Hayward’ kiwifruit treated with propylene continuously and for 48 h, fruit had a decrease in TA to eating levels, whereas other treatments did not reach the optimum eating range.
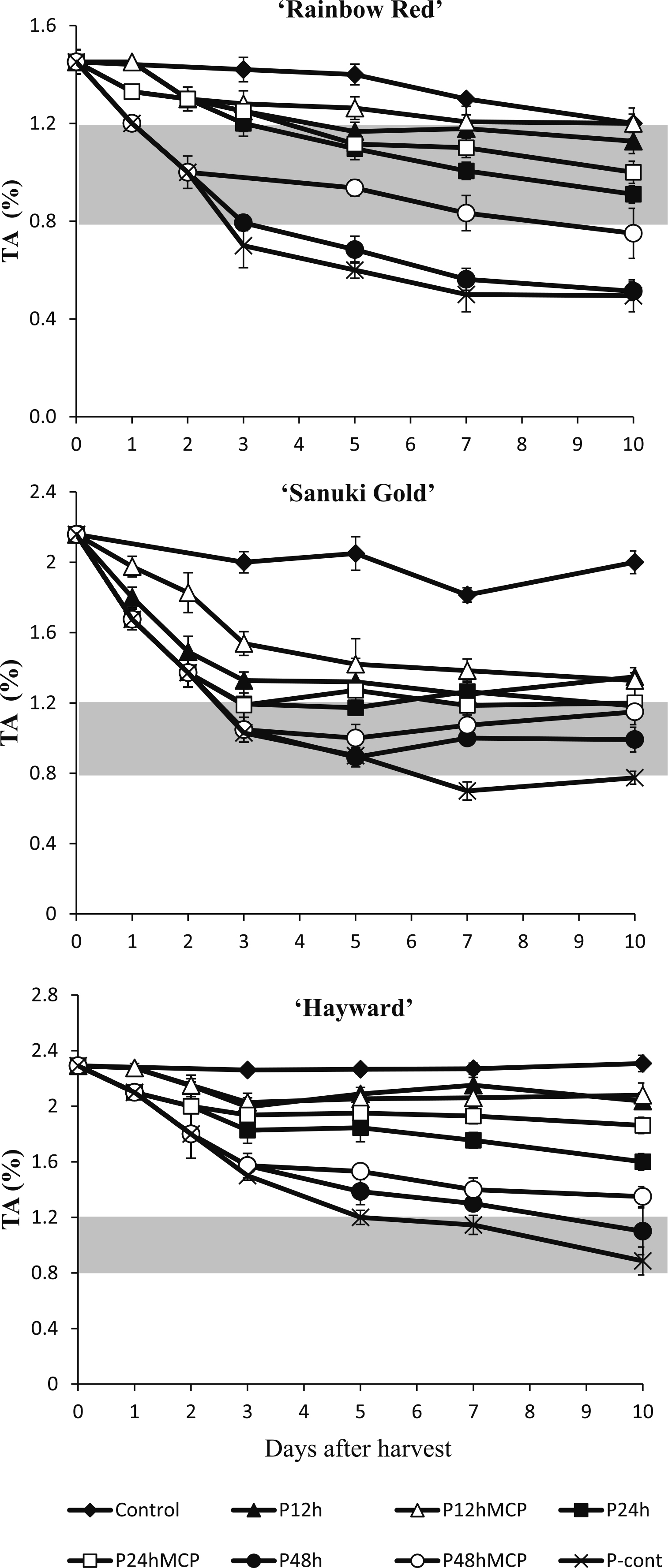
Effect of propylene and 1-MCP treatment on titratable acids (TA) of kiwifruit cultivars ‘Rainbow Red’, ‘Sanuki Gold’, and ‘Hayward’. Gray region indicates the optimum eating stage. TA is expressed as percentage (%) citric acid equivalents with each data point being from measurements of 5 fruit with SE bars.
Kiwifruit is a climacteric fruit and ethylene (or its analogue propylene) treatment induces fruit ripening (Antunes et al., 2000; Kim et al., 1999; Sfakiotakis et al., 1997; Yin et al., 2008, 2010). Atkinson et al. (2011) proposed a model of kiwifruit ripening induced by exogenous ethylene (see continuous lines in Fig. 6). It consists of four main phases, among which a remarkable decrease in fruit firmness occurs during Phase II before the induction of autocatalytic ethylene. During Phase III, kiwifruit softens to reach edible firmness together with the induction of endogenous ethylene production, while Phase IV ushers in the overripe stage. In this study, kiwifruit treated with propylene continuously had a drastic decrease in firmness within 3–5 days, with the commencement of a climacteric ethylene rise on days 5–7, illustrating that softening in kiwifruit occurs earlier before the beginning of endogenous ethylene production in the three kiwifruit cultivars studied (Figs. 2 and 3). The ability of ethylene/propylene treatment to induce a drastic decrease in firmness before the initiation of autocatalytic ethylene has similarly been observed in ‘Hayward’ (Atkinson et al., 2011; Taglienti et al., 2009; Yin et al., 2008), ‘Hort16A’ (Richardson et al., 2011), and ‘Sanuki Gold’ kiwifruit (Mworia et al., 2010, 2012). This suggests that kiwifruit exhibits a time lag between fruit softening and ethylene biosynthesis during ripening, unlike most climacteric fruit such as tomato, banana, pear, and avocado, in which softening is synchronized with the induction of endogenous ethylene. In this study, fruit treated continuously with propylene had a drastic decrease in firmness of about 80%, suggesting that Phase I and Phase II occurred within 3 days; as a result, fruit entered the “eating window”, indicating the beginning of Phase III (Figs. 3 and 6). The “eating window” of ‘Rainbow Red’ and ‘Sanuki Gold’ fruit was between 3 and 5 days while that of ‘Hayward’ was 3–10 days with continuous propylene treatment. This disparity in duration of the “eating window” and subsequent acquisition of ripening characteristics during the “eating window” can be attributed to cultivar differences. It is further suggested that, upon the induction of autocatalytic ethylene, the “eating window” is likely to be shortened, as demonstrated in ‘Rainbow Red’ and ‘Sanuki Gold’ kiwifruit.
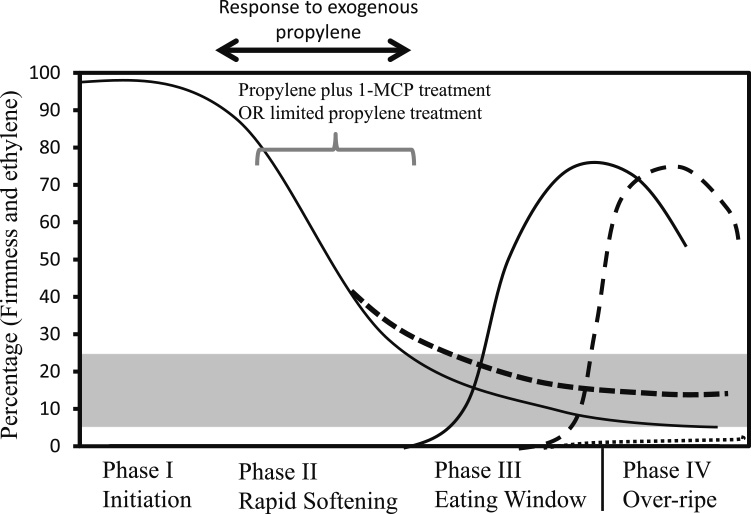
Schematic relationship between fruit firmness phases, ethylene production, and “eating window” of kiwifruit modified from Schröder and Atkinson (2006) and Atkinson et al. (2011). The continuous lines indicate proposed change in fruit firmness and ethylene production during ripening. The dashed line indicates modified ethylene production and softening after exposure to propylene or propylene plus 1-MCP treatment in kiwifruit. The dotted line indicates ethylene production after limited exposure to propylene treatment. Gray region depicts kiwifruit edible firmness range.
Interestingly, the treatment of kiwifruit with propylene for 24 h induced softening, an increase in SSC, and a decrease in TA for kiwifruit even without the detection of ethylene production in ‘Rainbow Red’ and ‘Hayward’, although a low level of ethylene was detected in ‘Sanuki Gold’ (Fig. 3). This signifies that the accumulation of cellular propylene during treatment triggers the kiwifruit softening process without inducing any detectable levels of ethylene, but upon its withdrawal, these softening processes are hampered. Richardson et al. (2011) demonstrated that, when ‘Hort16A’ kiwifruit was treated with ethylene for different durations of 12 h, 24 h, and 36 h, ethylene production was initiated earlier in kiwifruit treated for 36 h (after 1 day) than in fruit treated for 24 h (between 1 and 7 days), and no ethylene production was detected in fruit treated with ethylene for 12 h. Fruit treated with ethylene continuously had a drastic decrease in firmness compared with the case upon short exposure to ethylene for 12 h without the detection of ethylene. In the present study, fruit treated with propylene continuously and for 48 h reached the propylene threshold required to trigger complete fruit softening, whereas exposure to propylene for 12 h or 24 h induced limited softening, probably due to the absence of autocatalytic ethylene. Our study shows the importance of an effective duration of exposure to propylene in kiwifruit during the preclimacteric stage (Phase II) in order to induce ripening leading to the attainment of edible quality fruit, even before the initiation of autocatalytic ethylene, by treating fruit with propylene for a limited duration.
1-MCP has the ability to delay ripening in kiwifruit by suppressing ethylene perception signal and softening, as depicted in ‘Hayward’ kiwifruit (Kim et al., 2001) and ‘Sanuki Gold’ kiwifruit (Mworia et al., 2010). The effectiveness of 1-MCP in delaying ripening is attributed to its affinity to bind at the ethylene receptors, blocking downstream ethylene signaling (Sisler et al., 1996; Watkins, 2006). In our study, the application of 1-MCP after propylene treatment delayed the initiation and progression of ethylene biosynthesis and overall fruit ripening. 1-MCP extended the “eating window” of kiwifruit, especially in fruit treated with propylene for 48 h that had started ethylene biosynthesis in ‘Rainbow Red’ and ‘Sanuki Gold’. This suggests that, in kiwifruit, immediately after the commencement of propylene treatment, even before ethylene production is initiated, synthesis of cell-wall degrading enzymes is initiated, which induces subsequent fruit softening, whereas the application of 1-MCP delayed fruit softening induced by propylene. Similar effects of 1-MCP in delaying fruit softening after ethylene or propylene treatment have also been observed in melon (Nishiyama et al., 2007) and ‘La France’ pear (Kubo et al., 2003), where 1-MCP was effective in suppressing the expression of cell-wall modulating genes such as PG, βGal, EXP, and PL. Terasaki et al. (2013) using a non-destructive firmness method demonstrated that kiwifruit softening occurs in a biphasic manner and, whereas most softening occurs in the first stage, ethylene inhibitors are capable of delaying softening of the second stage (coinciding with the “eating window”), thereby delaying the time when fruit reach the overripe (senescence) stage. This illustrates that the application of 1-MCP after propylene treatment delays kiwifruit softening through the inhibition of ethylene signaling and has practical potential for extending the “eating window”, thus providing a prolonged shelf-life of kiwifruit in order to meet consumer demand (Boquete et al., 2004; Kim et al., 2001; Watkins, 2006).
The soluble solid content in kiwifruit increases with fruit ripening because of the conversion of stored starch to simple sugars (Jordan et al., 2000). Propylene treatment induced a dramatic increase in SSC and a decrease in TA depending on the duration of exposure in the three kiwifruit cultivars (Figs. 4 and 5). ‘Rainbow Red’ and ‘Sanuki Gold’ kiwifruit are classified as premium fruit having high SSC compared with ‘Hayward’ kiwifruit. Nishiyama et al. (2008) showed that A. chinensis cultivars such as ‘Rainbow Red’ and ‘Sanuki Gold’ had higher total sugar content than A. deliciosa such as ‘Hayward’, which was attributed to the predominance of glucose, fructose, and sucrose, which agrees with our observation. On the other hand, total TA decreased more rapidly in fruit treated continuously with propylene during ripening than in fruit treated for limited durations in the three kiwifruit cultivars. 1-MCP was effective in suppressing the decrease in TA after propylene treatment. High TA values were observed in ‘Hayward’ fruit compared with those in ‘Rainbow Red’ and ‘Sanuki Gold’ kiwifruit. In ‘Rainbow Red’ and ‘Sanuki Gold’ fruit treated with propylene continuously or for 48 h, the acidity levels were too low after day 7, so they were not suitable for consumption. Taking these findings together, the different durations of propylene treatment highlight the involvement of propylene in acquisition of the optimal edible stage among individual kiwifruit cultivars.
Attainment of optimum ripening highlights cultivar difference in kiwifruitDetermining the optimum ripening duration of ethylene (or propylene) treatment for specific kiwifruit cultivars is important for marketers and consumers. The response of kiwifruit to ethylene during ripening depends on the species, maturity stage, and cultivar (Mworia et al., 2010). Whereas current ripening protocols are largely based on ‘Hayward’ and ‘Hort16A’ kiwifruit, the introduction of new cultivars necessitates determination of the optimum edible stage in individual cultivars. For example, consumer preference for ‘Hayward’ kiwifruit is based on firmness of < 10 N, SSC > 12.5%, and TA < 1.17% (Crisosto and Crisosto, 2001), while in ‘Hort16A’ kiwifruit, firmness of 4.9–9.8 N, yellow color, and high SSC (16%) are most preferred (Patterson et al., 2003). In this study, cultivar differences were observed in the rate of softening, levels of SSC and TA, and the presence or absence of ethylene production during the period of the “eating window”. ‘Sanuki Gold’ and ‘Rainbow Red’ fruit treated with propylene continuously or for 48 h produced ethylene and ripened, while in ‘Hayward’, only fruit treated with propylene continuously produced ethylene (Fig. 2).
Fruit treated with propylene continuously softened to < 10 N, increased in SSC, and decreased in TA within 3 days in the three kiwifruit cultivars. The limiting feature of fruit treated with propylene continuously in ‘Rainbow Red’ and ‘Sanuki Gold’ kiwifruit was that fruit softened below the recommended level of firmness, reaching the overripe stage after day 5. ‘Hayward’ fruit treated with propylene continuously or for 48 h with or without 1-MCP reached edible firmness by day 3 and they had an extended “eating window”. Kiwifruit treated with propylene for 24 h in all cultivars achieved eating quality after 7 days and had the potential for a longer shelf-life without the detection of ethylene. Fruit treated with propylene for 12 h did not have the overall prerequisite fruit attributes for consumption because consumers prefer fruit with a softened core and outer pericarp, high SSC, and low TA. Notably, fruit treated with propylene for 48 h followed by 1-MCP in all three kiwifruit cultivars achieved suitable eating firmness with prolonged shelf-life of up to 10 days. Our study further indicates that kiwifruit treated with propylene for 48 h followed by 1-MCP treatment achieved optimum SSC and TA levels and this optimum condition was maintained up to day 10. Since the balance between SSC and TA contributes to taste, it consequently contributes to consumer acceptance of kiwifruit. For example, in ‘Hayward’ kiwifruit, it was shown that the degree to which consumers liked and accepted fruit with high SSC (> 12.5%) content was between 83.8% and 89.9%, whereas consumers disliked fruit with high TA (> 1.17%) because they had a sour taste (Crisosto and Crisosto, 2001). Notably, low levels of TA (< 0.8%) were attained in ‘Rainbow Red’ and ‘Sanuki Gold’ kiwifruit treated with propylene continuously; hence these fruit would not readily be accepted by consumers.
Our results clearly illustrate the importance of propylene exposure in inducing ripening and extending the “eating window” using 1-MCP in different kiwifruit cultivars. Two options are viable in prolonging the “eating window” of kiwifruit. First, judicial pretreatment with ethylene/propylene for a limited time would induce ripening attributes without inducing endogenous ethylene, so fruit can maintain the “eating window” for a long time. Secondly, the application of 1-MCP after prolonged ethylene/propylene treatment has the ability to extend the shelf-life even after the initiation of ethylene, as depicted in ‘Rainbow Red’ and ‘Sanuki Gold’ fruit. We recommend propylene treatment for 48 h followed by 1-MCP treatment to obtain the best ready-to-eat fruit in terms of fruit softening, increase of SSC, and reduction of TA for ‘Rainbow Red’ and ‘Sanuki Gold’ kiwifruit. ‘Hayward’ fruit treated with propylene continuously or for 48 h attained the best consumption quality with low TA, making the fruit desirable. Therefore, on the basis of the four phases of kiwifruit ripening (Atkinson et al., 2011), Phase III provides the optimum “eating window” and extending Phase III by limited propylene treatment or the use of ethylene inhibitors such as 1-MCP is a viable way to ensure a longer shelf-life in kiwifruit (Fig. 6). This information is important for the practical handling of kiwifruit during shipping and marketing. Proper scheduling of ethylene/propylene and 1-MCP treatment depending on consumer fruit preference and market demand can be achieved by using a suitable ripening protocol to provide premium-quality fruit to consumers in a timely manner. Furthermore, 1-MCP treatment proved to be a suitable option in extending the optimum quality of kiwifruit during the ripening process in situations where kiwifruit is intended for longer shelf-life.
In conclusion, limited propylene treatment or propylene treatment followed by 1-MCP treatment extends the edible stage of ‘Rainbow Red’ and ‘Sanuki Gold’ kiwifruit. Conversely, ‘Hayward’ kiwifruit had a longer shelf-life after propylene treatment even without 1-MCP treatment. This highlights cultivar differences in kiwifruit necessitating the determination of optimum ethylene (propylene) regimes to induce fruit ripening without endogenous ethylene. Upon registration in Japan, 1-MCP will be instrumental in postharvest handling in order to extend the shelf-life of kiwifruit. Taken together, this information is useful in determining appropriate ethylene (propylene) treatment regimes together with 1-MCP treatment in order to obtain high-quality fruit and effectively extend the postharvest shelf-life of kiwifruit.