2021 年 90 巻 4 号 p. 460-468
2021 年 90 巻 4 号 p. 460-468
Cut dahlia (Dahlia Cav.) flowers have recently become popular in Japan, but their marketability is limited by their poor vase life. During the postharvest period, the senescence of petals initiates from the outermost whorl and then proceeds into the inner petals. We found that the drawing resistances of petals in the dahlia ‘Kokuchou’, ‘Nesshou’, and ‘Saisetsu’ decreases with time because the petals gradually detach from the ovaries. A reduction in drawing resistance occurred prior to the end of vase life. Exogenous ethylene treatment of ‘Saisetsu’ cut inflorescences accelerated the decline of the petal drawing resistance and thus shortened the vase life. Ethylene production by individual florets in cut inflorescences began before or on the day of harvest. Simultaneously, abscission layers developed at the petal-ovary boundaries of the outermost florets. The florets without ovaries, which did not form the abscission layer, had a longer life than the florets with ovaries. Furthermore, we observed a marked rise in petal sugar contents in florets without ovaries compared to those with ovaries when maintained in 2% fructose. These results indicate that ethylene-promoted abscission layer development directs the course of petal senescence, which is characterized by wilting and discoloration due to a blocking of water flow and carbohydrates into the petals.
Dahlia (Dahlia Cav.), which forms tuberous roots, is an important ornamental plant and is widely grown as a cut flower, a garden ornamental, or as a potted plant. Dahlia cultivars have become popular cut flowers in Japan in recent years. However, the vase life, which is affected by deterioration processes common among cultivars (Onozaki and Azuma, 2019), is a week or less, which is relatively short compared with other members of Asteraceae. Similar to chrysanthemums, dahlia inflorescences are capitula consisting of many floret whorls. As is the case with some chrysanthemums (Van Geest et al., 2017), the symptoms of senescence in dahlias are petal wilting and discoloration that first occur in the outer florets and then progress into the inner petals (Shimizu-Yumoto and Ichimura, 2013).
There are many reports of cut flower senescence induced by endogenous ethylene production, although the sensitivity and reaction to ethylene vary among species (Ichimura et al., 1998). The cut inflorescences of dahlias have been shown to continuously generate ethylene during the postharvest period (Azuma et al., 2020; Shimizu-Yumoto and Ichimura, 2013). However, the ethylene responses in dahlias are considered to be insensitive or mildly sensitive (Dole et al., 2009; Shimizu-Yumoto and Ichimura, 2013), and a pulsing treatment with STS after harvest had little effect on prolonging vase life (Shimizu-Yumoto and Ichimura, 2013).
On the other hand, petal abscission is also a typical characteristic of cut flower senescence. The process of abscission involves cell separation in the abscission layer that forms at a predetermined site of the organ that is ultimately shed. Anatomical observations in combination with measurements of the force required to remove the organ could help quantify the process (Craker and Abeles, 1969). In cut dahlias, however, petal abscission is not as severe due to the long bracts that hold the petals tightly. In other cut flowers, abscission layer formation commonly causes fatal disorders that are detrimental to the ornamental value and hence the vase life. In sunflowers (Helianthus annuus L.), which are classified into the same subtribe (Helianthodae) as dahlias, abscission layer formation causes the petals to wilt and drop, thus ending the vase life (Tata and Wien, 2014). The formation of abscission layers can be triggered by aging, environmental factors, and phytohormones (Estornell et al., 2013; Taylor and Whitelaw, 2001). Among such phytohormones, endogenous ethylene is considered to be the main factor regulating abscission layer formation (González-Carranza and Roberts, 2012; Kim, 2014; Nakano and Ito, 2013). Although some researchers have demonstrated a relation between ethylene and flower senescence in dahlias, there has been little attention paid to petal abscission.
We hypothesized that the senescence of cut dahlia inflorescences, which is characterized by petal discoloration and desiccation, may be caused by ethylene-sensitive abscission layer formation in petals and that the development of the abscission layer blocks the flow of water and carbohydrates into the petals. In this study, we tested this hypothesis by focusing on 1) abscission layer development and petal senescence in dahlia florets and 2) the flow of water and carbohydrates into the petals.
Dahlia ‘Kokuchou’, ‘Nesshou’, and ‘Saisetsu’ were propagated by cutting and grown according to a standard procedure in an open field at Kyoto University in 2019 and 2020. Cut inflorescences were harvested from May to November once the inflorescences had reached the standard point of commercial maturity; that is, the horizontal expansion of the outermost petals. The cut inflorescences were recut under water leaving 28–30 cm stems and then placed in deionized water containing 1 mL·L−1 KathonTM CG (Rohm and Haas Japan K. K., Tokyo, Japan) containing 11.3 g·L−1 5-chloro-2-methyl-4-isothiazolin-3-oneas and 3.9 g·L−1 2-methyl-4-isothiazolin-3-one as a bactericide. The vase life of the cut inflorescences was considered to have terminated when the florets in the first and the second wholes were wilted or discolored.
Postharvest environmental conditions were controlled at 20°C, 12 h lighting by day-white fluorescent tubes, and 50%–60% RH.
Exp. 1. Drawing resistances of florets from inflorescencesWe constructed a device to measure the drawing (or pull) resistance of the petals from an inflorescence. A clip attached to a petal was connected by a thread to a digital force gauge (FGP-0.5; Nidec Corporation, Tokyo, Japan) (Fig. 1A). The inflorescence was pulled slowly on a smooth plastic plate to measure the peak force when the petal was separated from the receptacle or the ovary. The drawing resistance of florets from different whorls in an inflorescence was measured on day 0, 1, 3, and 5 after harvest.

The device for measuring drawing resistances (A), and drawing resistances of petals from ‘Kokuchou’ (B), ‘Nesshou’ (C), and ‘Saisetsu’ (D) inflorescences. Different letters indicate significant differences within the same whorls by Tukey’s test (P < 0.05). Bars indicate SEs (n = 6).
‘Saisetsu’ inflorescences harvested in August were placed in a glass chamber and continuously exposed to exogenous ethylene at 1 μL·L−1 for 18 h at room temperature (approximately 25°C). The cut inflorescences were then moved to a growth chamber maintained at 20°C and 12 h lighting to evaluate the vase life after the ethylene treatment. The drawing resistance of the petals in different whorls was measured as described in Exp. 1. on day 2 after harvest.
Exp. 3. Ethylene production of individual floretsThe cut inflorescences were harvested in July and August. Ethylene production of the individual florets was measured on day 0, 1, 3, and 5 after harvest. The florets with ovaries, but without bracts in the first (outermost), third, and fifth whorls were dissected from the cut inflorescences on the day of measurements and weighed. The florets were sealed in a 25-mL glass vessel for 1 hour, after which 1 mL of headspace gas was injected into a gas chromatography apparatus (GC-15A; Shimadzu Corporation, Kyoto, Japan) fitted with an FID detector.
To elucidate the pre-harvest ethylene production, florets were dissected from the ‘Saisetsu’ inflorescences following the early developmental stages from intact plants growing in the open field in September 2019 (Fig. 4A). Stage A: the outermost petals just protruded from the bracts, but only expanded slightly; Stage B: the outermost petals began to expand; Stage C: the outermost petals expanded to about 45°; Stage D: the outermost petals expanded horizontally (the standard harvest stage). The ethylene production was measured by gas chromatography as described above.
Exp. 4. Senescence of individual florets Exp. 4.1 Individual floret life and fresh weight changesFlorets, with or without ovaries, were dissected from the first, third, and fifth whorls of an inflorescence. The dissected florets were then held in 1.5-mL microtubes containing deionized water or 2% fructose solution. Both of the vase solutions contained 1 mL·L−1 KathonTM CG as a bactericide. Each treatment consisted of 12–15 florets collected from 12 cut inflorescences. The life of the florets was defined as the number of days until a quarter of the petal area appeared withered or discolored, as measured under 20°C and 12 h lighting.
The fresh weights (FW) of the first and fifth florets in ‘Kokuchou’ were recorded on day 0, 3, 4, and 6.
Exp. 4.2 Abscission layer formation in the floretsThe outermost florets, with or without ovaries, were sampled on day 0 and day 6 after harvest and cut into 3-mm segments containing the petal-ovary boundaries. The samples were fixed using FAA (ethanol: water: formaldehyde: acetic acid = 12:6:1:1), and then dehydrated using an ethanol series at 30%, 50%, 70%, 80%, 90%, 95%, 99.9%, and 100% ethanol. The prepared samples were embedded in resin (Technovit 7100 kit; Kulzer, Germany) in order to obtain 5-μm-thick sections using a rotary microtome (RM2155; Leica, Germany). The sections were stained using Delafield’s hematoxylin for 30 min and then observed under a microscope (BX-53; Olympus Corporation, Tokyo, Japan). Then, photographs were taken with a digital camera (DP74; Olympus Corporation). We also treated the florets with 1 μL·L−1 ethylene for 24 h on day 0. They were then sampled on day 5 and resin sections were prepared for observation.
Exp. 4.3 Carbohydrate flow into petals‘Kokuchou’ florets were dissected from the first whorls, with or without ovaries. Two treatment media were prepared. One treatment consisted of Gelrite medium to which 1 mL·L−1 KathonTM CG (as a bactericide) and 2% fructose were added. The other treatment did not include any fructose. In each treatment, only 1 mm of the basal portion was immersed in the treatment medium. Each treatment consisted of eight florets collected from four cut inflorescences.
The soluble carbohydrates were extracted from the petals following the protocol modified from Ichimura and Hisamatsu (1999). The petals (0.2–0.3 gFW) were frozen in liquid nitrogen and ground into pieces. The petal pieces were immersed in 4 mL 80% ethanol and then heated in a water bath at 75°C for 20 min. The samples were centrifuged at 9,000 rpm for 5 min, after which the supernatants were heated below 70°C until dried. The pellets were resolved in 1 mL deionized water and then centrifuged at 12,000 rpm for 5 min. The supernatants were collected and used for analysis by high performance liquid chromatography (HPLC).
Carbohydrates were separated using an HPLC system (LC-10A; Shimadzu) equipped with a refractive index detector (RID-10A; Shimadzu) on a column (SUGAR SC1011; Showa Denko K. K., Tokyo, Japan) kept at 80°C. Carbohydrates were eluted with 5% acetonitrile at a flow rate of 1 mL·min−1. The identity of each peak was confirmed using authentic carbohydrates.
Statistical analysisData were subjected to analysis of variance, t-test and Tukey’s test using Costat software (v.6.4, Cohort Software, UK).
Petal wilting of cut ‘Saisetsu’ inflorescences held in deionized water was evident in the outermost florets on day 3 after harvest; these inflorescences had the shortest vase life (4.3 days) among the three cultivars. The vase lives of ‘Nesshou’ and ‘Kokuchou’ were 7.5 days and 9.5 days, respectively (data not shown).
At the standard harvest stage (day 0), the petals were detached with ovaries from the receptacles, and the inner petals could be drawn out more easily than the outer petals. On day 3, the petals of the florets in the first (outermost) whorl (hereinafter referred to as the first petals or florets) were drawn out not from the receptacles, but from the ovaries, and parts of the inner petals in the fifth and sixth whorls were drawn out from the receptacles. On day 5, the petals of ‘Nesshou’ and ‘Saisetsu’ were all drawn out from the ovaries. On the other hand, parts of the inner petals were still drawn out from the receptacles in ‘Kokuchou’ on day 5 (data not shown).
The drawing resistance of the outer petals decreased with time. In ‘Kokuchou’, the petal drawing resistance in all the whorls showed little change on day 1. On day 3, drawing resistance decreased a little in the first petals, and increased a little in the fifth and sixth petals. On day 5, the drawing resistance greatly decreased in the first petals, but showed no marked decline in the inner ones compared with day 0 (Fig. 1B).
In ‘Nesshou’ and ‘Saisetsu’, the drawing resistance of the first petals decreased greatly on day 3, and they could be drawn out more easily than the inner petals. However, the drawing resistance of inner petals showed no significant decline in ‘Nesshou’, but declined significantly in ‘Saisetsu’ on day 3. On day 5, the drawing resistance of the inner petals also decreased in ‘Nesshou’. In ‘Saisetsu’, the outermost petals had wilted on day 5, and the petal drawing resistance reached low levels in all the whorls as compared with ‘Nesshou’ (Fig. 1C, D).
Exp. 2. Effects of exposure of cut inflorescences to exogenous ethyleneThe vase life of ‘Saisetsu’ inflorescences was 4.2 days for the control and 2.8 days for 1 μL·L−1 exogenous ethylene-treated inflorescences (Table 1). The drawing resistance of petals on day 2 declined significantly in all the whorls in ethylene-treated inflorescences compared to those without ethylene treatment (Fig. 2A). The outer petals in the first to fourth whorls in cut inflorescences treated with ethylene showed earlier wilting and necrotic browning on day 5 as compared with the control. However, no wilting or discoloration were observed in the petals further in than the fifth whorls on day 5, even in the ethylene-treated inflorescences (Fig. 2B).

Effects of exposure to 1 μL·L−1 ethylene on the vase life of cut ‘Saisetsu’ inflorescences.

Drawing resistances of petals (A) and the petal senescence (B) of ‘Saisetsu’ cut inflorescences treated with or without 1 μL·L−1 ethylene for 18 h. Different letters indicate significant differences within the same whorls by Tukey’s test (P < 0.05). Bars indicate SEs. The photograph was taken on day 5.
At the standard harvest stage (day 0), the dahlia inflorescences had begun to generate ethylene, and the highest ethylene production by the first (outermost) florets was detected. In ‘Kokuchou’ and ‘Nesshou’ the ethylene production by the first florets dropped significantly on day 1 and remained constant during the postharvest period. In the third and fifth florets, ethylene production rose on day 3 and then fell slightly on day 5 (Fig. 3A, B). In ‘Saisetsu’, the ethylene production by individual florets rose in all the whorls measured on day 1 and then declined on day 3 in the first florets, but remained high in the third and fifth florets (Fig. 3C).
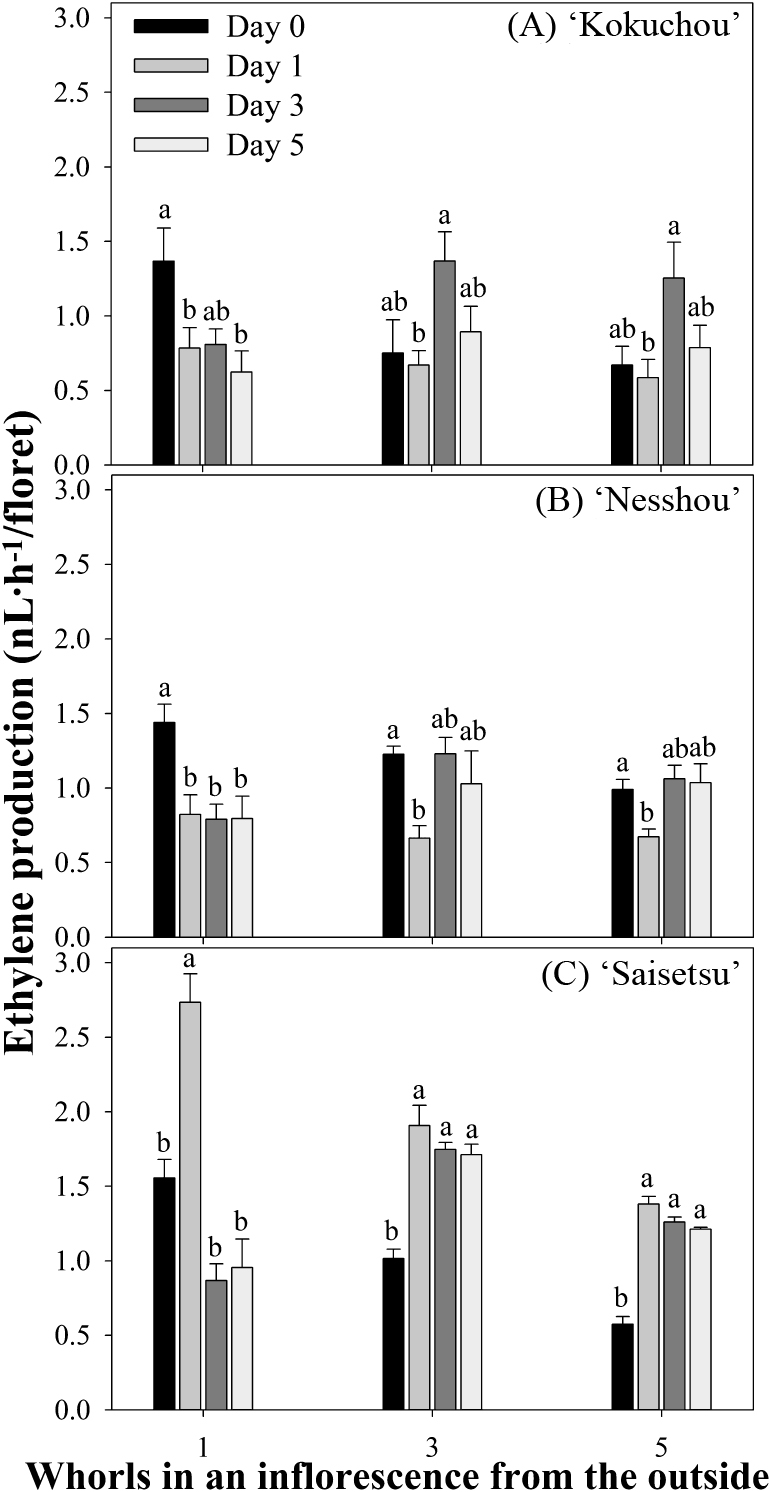
Ethylene production by individual ‘Kokuchou’ (A), ‘Nesshou’ (B), and ‘Saisetsu’ (C) florets in the cut inflorescences. Different letters indicate significant differences within the same whorls by Tukey’s test (P < 0.05). Bars indicate SEs (n = 4).
In the ‘Saisetsu’ florets, ethylene production had begun at the early developmental stages A–D before harvest (Fig. 4A). The ethylene production by individual florets rose at stage D, and the outer florets produced more ethylene than the inner florets (Fig. 4B).

The pre-harvest inflorescence stages of ‘Saisetsu’ growing in the open field (A) and the ethylene production by the individual florets at stage A–D (B). Different letters indicate significant differences within the same whorls by Tukey’s test (P < 0.05). Bars indicate SEs (n = 4). Stage A: the outermost petals protruded from the bracts, but only expanded slightly; Stage B: the outermost petals began to expand; Stage C: the outermost petals expanded to about 45°; Stage D: the outermost petals expanded horizontally (the standard harvest stage).
To measure the individual floret life, we dissected florets from the first, third, and fifth whorls of the inflorescences harvested at standard maturity. In ‘Kokuchou’ and ‘Nesshou’ inflorescences, the florets without ovaries had a longer vase life than the florets with ovaries. In ‘Saisetsu’ inflorescences, however, there were no significant differences between the florets with ovaries and those without. Treatment with 2% fructose significantly prolonged the floret life in all cultivars (Table 2). The florets from the outer whorls wilted earlier than those from the inner whorls.

Effects of ovary removal and fructose treatments on the life of ‘Kokuchou’, ‘Nesshou’, and ‘Saisetsu’ florets.
In the first florets placed in deionized water, the relative FW of florets with ovaries increased to a limited extent on day 3 and day 4 compared with those without ovaries. On day 6, the relative FW of florets with ovaries declined significantly compared with florets in the other treatments. In the florets held in 2% fructose, the relative FW of florets without ovaries increased markedly on day 3 compared with the florets with ovaries. On day 4, the relative FW of florets with ovaries began to decrease, whereas the FW of florets without ovaries did not. On day 6, the florets without ovaries had a significantly higher FW compared to those with ovaries (Fig. 5A).

Effects of ovary removal on the changes in the relative fresh weight of ‘Kokuchou’ florets dissected from the first (A) and fifth whorls (B) of inflorescences and placed in DIW or 2% fructose solution. Bars indicate SEs (n = 8).
Among the fifth florets placed in DIW or 2% fructose, the relative FW of the florets without ovaries held in fructose solution exhibited the greatest increases on day 3 and day 4 and a slight decrease on day 6 (Fig. 5B), although the relative FW of the florets with ovaries steadily increased throughout the six days.
Exp. 4.2 Abscission layer formation in the floretsMicroscopical photographs of longitudinal sections of the outermost florets of ‘Kokuchou’, ‘Nesshou’, and ‘Saisetsu’ are shown in Figure 6. Abscission cells were observed at the petal base boundary even at the standard harvest stage (Fig. 6A, B, C). After being placed in deionized water for 6 days, florets with ovaries had clear abscission layers, which exhibited some break-down at the petal base (Fig. 6D, E, F). On the other hand, florets without ovaries did not develop abscission zones at the petal bases (Fig. 6G, H, I). In our preliminary experiment using ‘Nesshou’, pre-harvest abscission layer formation in the first florets was observed at inflorescence stage B (Fig. S1).

Resin sections of the florets from the first whorl on day 0 (A, B, C), and florets with ovaries (D, E, F) or without ovaries (G, H, I) on day 6. Frames show the abscission layers. O: ovaries, P: petals, C: calyxes. Scale bars indicate 200 μm. Note that a breakdown of the abscission zones occurred in ‘Nesshou’ (E).
In the florets treated with 1 μL·L−1 ethylene for 24 h on the day of harvest, abscission layer development and petal separation from the ovary were accelerated and more obvious on day 5 (Fig. S2).
Exp. 4.3 Carbohydrate contents in petalsThe main sugars detected in petals were glucose and fructose, although a relatively low sucrose content was detected. In addition to these, two unidentified HPLC peaks, which changed little after five days, were also detected. Irrespective of the presence of ovaries, the sugar contents decreased significantly in the florets placed in the medium without fructose for five days (Table 3). In the florets placed in 2% fructose, those with ovaries had a slightly decreased sugar content on day 5, whereas those without ovaries exhibited a slightly increased sugar content compared with day 0; in particular, the fructose and total sugar contents increased in the florets without ovaries after being placed in 2% fructose.

Effects of ovary removal on the sugar content of ‘Kokuchou’ petals held in DIW or 2% fructose medium.
In each of the dahlia cultivars, the senescence of cut inflorescences was accompanied by a decline in petal drawing resistance during the vase period (Fig. 1B, C, D). There are four stages in flower organ abscission: the first stage is marked by differentiation of the abscission zone, the second stage by response to ethylene signals, and the third by the increase of organ break strength which results from cell wall loosening and degradation. In the fourth and last stage, the separation of organs from the plant body is complete, and a protective layer is formed (Bleecker and Patterson, 1997; Kim, 2014; Roberts et al., 2002; Taylor and Whitelaw, 2001). In this study, we quantified the timing and degree of abscission layer formation in the individual florets by measuring the petal drawing resistance.
Although the petal drawing resistance was high at harvest, a decrease in petal drawing resistance occurred more rapidly in the outer florets than in the inner ones, which correlated to the sequence of petal senescence in inflorescences. Once the outer petals had separated from the ovaries, they could be drawn out easily from the inflorescences. Notably, even when the petals were fully separated from the ovaries and showing signs of wilting, they did not drop from the inflorescences, because the bracts held the petals tightly.
The speed of abscission layer development in flowers often correlates with vase life. In sunflowers (family Asteraceae), abscission leads to petal dropping and vase life termination; short-lived cultivars show an earlier decline in petal drawing force (Tata and Wien, 2014). Onozaki and Azuma (2019) also demonstrated that dahlia lines with a petal abscission (dropping) pattern of senescence only appeared in the initial generations of longevity breeding. In the present study, ‘Saisetsu’ showed a significantly shorter vase life than ‘Kokuchou’ and ‘Nesshou’. The decline in petal drawing resistance in ‘Saisetsu’ was also more rapid and more pronounced than in the other two cultivars. In ‘Nesshou’, which had a medium length vase life among the three cultivars, the decrease in drawing resistance of the outer petals was significantly larger than the inner ones. In ‘Kokuchou’, which had the longest vase life, the decline in drawing resistance in the outer petals was relatively slow, and the inner petals showed no significant decline in drawing resistance during the vase period (Fig. 1B, C, D). These results may indicate that the progress of abscission layer development from the outer florets to the inner ones was coincident with inflorescence life.
Cut flower senescence is generally characterized by wilting or abscission (Van Doorn and Woltering, 2008; Woltering and Van Doorn, 1988). Van Doorn (2001) identified four categories of flower life cessation: 1) ethylene-sensitive petal wilting, 2) ethylene-insensitive petal wilting, 3) ethylene-sensitive petal abscission, and 4) ethylene-insensitive petal abscission. In the last two types of senescence, the petals remain turgid and do not wilt during abscission.
To elucidate the relation between ethylene and petal abscission, the ‘Saisetsu’ cut inflorescences were treated with 1 μL·L−1 exogenous ethylene for 18 h. Compared with the control, the cut inflorescences treated with exogenous ethylene showed wilting of the outer petals and the end of vase life was reached on day 3 after harvest (Table 1). Exogenous ethylene treatment also caused a sharp decline in petal drawing resistance in all the whorls on day 2 (Fig. 2A). Although the drawing resistance declined on day 2, the inner petals in the fifth and sixth whorl did not show severe wilting on day 5, even in the inflorescences treated with ethylene (Fig. 2B).
Several studies have focused on the effects of ethylene on the senescence of dahlia inflorescences (Dole et al., 2009; Shimizu-Yumoto and Ichimura, 2013; Woltering and Van Doorn, 1988); however, the relation between ethylene and petal abscission is seldom mentioned. The results of our experiment revealed that ‘Saisetsu’ is sensitive to ethylene and that exogenous ethylene does not cause immediate petal wilting, but rather abscission of petals from the ovaries. Based on these results, we can conclude that the senescence pattern of dahlia is category 3 (ethylene-sensitive petal abscission, Van Doorn, 2001).
We found that the cut inflorescences harvested in summer had started to generate endogenous ethylene from the first, third, and fifth florets at the standard harvest stage. During the postharvest period, the inflorescences released ethylene continuously, generating it from the outermost florets to the inner ones (Fig. 3). In ‘Saisetsu’, which had the shortest vase life and the most rapid decrease in petal drawing resistance, the endogenous ethylene could be detected from the florets at the petal-expanding stages of inflorescences before harvest (Fig. 4).
We also treated the ‘Saisetsu’ inflorescences at stage C with 1 μL·L−1 1-MCP in the field and they were harvested at standard maturity. The petal drawing resistance was higher in the inflorescences treated with 1-MCP than those untreated (Fig. S3). The vase life of cut inflorescences with pre-harvest 1-MCP treatment was also extended a little; Shimizu-Yumoto and Ichimura (2013) found the same results by postharvest exposure with 1-MCP. This extension may be due to 1-MCP lightening the abscission layer development promoted by endogenous ethylene.
We presumed the presence of ovaries, which had a high ethylene production rate, would induce development of the abscission layer and earlier senescence in individual florets. The florets without ovaries had a longer life than those with ovaries in the holding solutions. The 2% fructose treatment extended the floret life effectively, irrespective of the floret position (Table 2).
Relative FW is a parameter reflecting the flow of water to petals. In the first florets, held in deionized water, florets with ovaries showed a relatively poor increase in FW compared with ovary-dissected florets, in which the abscission layers did not develop, and the FW decreased markedly on day 6. These results may be due to poor water uptake of petals caused by abscission layer formation. The same trend was found in florets held in 2% fructose: the relative FW in florets without ovaries increased to a much greater degree than in the florets with ovaries. On day 6, those florets without ovaries and placed in 2% fructose showed the highest FW values among all the treatment groups, indicating that these florets were better able to absorb water and sugars than those with ovaries (Fig. 5A).
The fifth florets showed a greater elevation in relative FW compared with the first florets, and this is likely due to the development of the abscission layer occurring later in the younger florets. During the initial period of postharvest, the fifth florets were able to uptake solutions to maintain FW. The florets held in 2% fructose showed a large rise in relative FW (Fig. 5B), presumably due to the increased sugar concentration, which plays a role in osmosis. The presence of highly soluble carbohydrates in flower buds has been shown to improve water uptake and vase life in some species of cut flowers (Eason et al., 1997; Norikoshi et al., 2016; Shimizu and Ichimura, 2005).
Microscopical observations of longitudinal sections of the outermost florets dissected from an inflorescence at the harvest stages showed that there were anatomically distinct cell layers in the petal-ovary boundaries (Fig. 6A, B, C). These cell layers, which consisted of smaller-sized cells compared with other petal cells, were similar to the petal abscission zone in other species (Patterson, 2001; Patterson and Bleecker, 2004; Van Doorn and Stead, 1997). Because the endogenous ethylene production of individual florets had initiated before the standard harvest stage, the abscission layers found in florets on day 0 may have been initiated at pre-harvest stages. In the preliminary studies, we observed abscission layers in stage B (Fig. S1).
In the florets with ovaries, the abscission layers had developed completely on day 6 and was somewhat degraded (Fig. 6D, E, F). In contrast, abscission layers were not found in the florets without ovaries (Fig. 6G, H, I), which are the main organs that produce ethylene (Azuma et al., 2020; Shimizu-Yumoto and Ichimura, 2013).
The results of the HPLC analysis were consistent with those reported in the literature; in particular, that the main carbohydrates in dahlia petals were glucose and fructose, and that the carbohydrate contents in petals decreased with time in the postharvest period without exogenous sugar supply (Azuma et al., 2019; Shimizu-Yumoto et al., 2020). The presence of an abscission layer on the petal-ovary boundaries significantly affected the water and carbohydrate flows into the petals. The content of fructose, glucose, and sucrose in the petals sharply decreased when the florets were held in a medium without sugars. In the florets held in 2% fructose, the petal sugar contents of florets with ovaries were reduced compared to day 0. On the other hand, those florets without ovaries were found to have significantly increased fructose, sucrose, and total sugar contents even on day 5 (Table 3).
The results of our anatomical observations and HPLC analyses of dahlia florets suggest that the abscission layers at the petal-ovary boundaries obstructed the water and carbohydrate flows into petals and caused the loss of petal FW and carbohydrate contents, even in intact inflorescences.
In conclusion, the senescence of dahlia petals is due to the water and carbohydrate deficiency caused by the development of an abscission layer at the petal-ovary boundaries. This development is promoted by ethylene that is generated from the outer florets initially and subsequently by the inner florets. The decrease in the petal drawing resistance is coincident with abscission layer development and may be a useful index for breeding dahlia cultivars with improved longevity.