2022 Volume 91 Issue 3 Pages 399-407
2022 Volume 91 Issue 3 Pages 399-407
The lily MYB12 gene, a positive regulator of anthocyanin biosynthesis, is targeted by microRNA828 (miR828). In bicolor tepals of Asiatic hybrid lilies with white lower halves and pigmented upper halves, accumulation levels of miR828 are higher in the lower halves than in the upper halves, and action of MYB12 is suppressed in the lower halves, resulting in bicolor tepal development. This is a newly identified mechanism of color pattern development in flowers. However, which wild species has donated the miR828-mediated bicolor tepal traits to these hybrid lilies is uncertain, and whether miR828-dependent pattern development occurs in species other than Lilium and is responsible for other types of color patterns is unknown. In this study, miR828 accumulation levels were compared between anthocyanin pigmented and unpigmented regions of flowers in lilies and other species. Lilium dauricum is among the parental wild species of Asiatic hybrid lilies. Lilium dauricum showed bicolor tepals, in which anthocyanins highly accumulated in the upper halves, and miR828 accumulation was more than 10 times higher in the lower halves than in the upper halves. Thus, the profile of miR828 accumulation was similar to that found in bicolor cultivars of Asiatic hybrid lilies. It is possible that the miR828-mediated bicolor tepal trait in Asiatic hybrid lilies is derived from L. dauricum. In L. cernuum var. album and an Oriental hybrid lily ‘Dizzy’, the suppression of MYB12 expression causes unpigmented tepals or tepal regions, but the unpigmented regions are spatially different from those in bicolor tepals of Asiatic hybrid lilies. MiR828 accumulation levels were similar between white and pink flowers of L. cernuum, and rather higher in pigmented regions than white regions of ‘Dizzy’ tepals, suggesting little involvement of miR828 in MYB12 expression suppression. MiR828 accumulation levels were evaluated in bicolor flowers of cherry sage, tulip, and Alstroemeria, but differences in miR828 accumulation were not detected between red and white petal/tepal regions, indicating that the mechanisms by which the bicolor flowers developed in these species are likely different from that occurring in Asiatic hybrid lilies and L. dauricum. Thus, the miR828/R2R3-MYB module is likely responsible for color only in lily flowers and only for the color pattern that consists of lower un-pigmented and upper pigmented regions.
Many kinds of color patterns, including spots, stripes, venations, bud-blushes, and bicoloration (two colors in single petals/tepals), often appear in flowers. Flower color patterns play critical roles in plant–pollinator communications because they affect pollinator preferences and their changes often cause pollinator shifts (Davies et al., 2012; Glover et al., 2013; Martins et al., 2017; Yuan et al., 2014). In addition, creating new flower color patterns is valuable for practical reasons because modified color patterns in floral organs can increase commercial value (Nakayama, 2014). Thus, the regulation of color pattern development is of great interest to plant biologists and breeders. Color patterns are produced by spatially and temporally restricted deposition of pigments; anthocyanin pigments are frequently involved in pattern development. Several mechanisms of restricted anthocyanin deposition have been identified in flowers. The major mechanisms are post-transcriptional gene silencing of chalcone synthase genes (Koseki et al., 2005; Morita et al., 2012; Ohno et al., 2011), the insertion of transposable elements into anthocyanin biosynthesis genes (Momose et al., 2013; Uchiyama et al., 2013), and spatially distinct expression of R2R3-MYB positive regulators in single petals (Albert et al., 2011; Hsu et al., 2015). The latter mechanism involves subgroup 6 members of R2R3-MYB transcription factors in many species, and subgroup 5 members in orchids, positively regulating anthocyanin biosynthesis (the grouping of R2R3-MYBs is according to Stracke et al., 2001); single plant species often express some of these positive regulators. Each of them exhibits temporally and spatially distinct expression profiles on petals, resulting in restricted pigment deposition depending on which R2R3-MYB genes each plant possesses. In addition to these mechanisms, microRNA-mediated mechanisms have recently been shown in lilies. In Asiatic hybrid lilies (Lilium spp.), microRNA828 (miR828) directly targets the subgroup 6 members of R2R3-MYB genes and accumulation levels of miR828 are spatially different on single tepals, and higher in lower halves than in upper halves. Then, the action of MYB12, the R2R3-MYB positive regulator, is suppressed in the lower halves, resulting in the creation of unpigmented regions (bicoloration, Yamagishi and Sakai, 2020). As miR828 is one of the highly conserved microRNAs among plants and is found in many monocot and eudicot species (Guan et al., 2014; Li and Lu, 2014; Roy et al., 2016), the miR828/R2R3-MYB module mediating pattern formation is expected to occur in other types of color patterns and in other species. However, the accumulation profiles of miR828 in other flower color patterns and in species other than Lilium remain unknown.
Lilies (Lilium spp.) are among the most important floricultural plants worldwide. Interspecific hybridization is the principal method of lily breeding. Wild lily species are classified into sections and species belonging to the same section exhibit relatively high seed production ability upon interspecific hybridization. Thus, cultivars are primarily developed from crosses of species within a single section. Asiatic hybrid lilies are derived from crosses among species belonging to a Sinomartagon/Daurolirion section (although Sinomartagon and Daurolirion were formerly classified as different sections, their close relationship is indicated by molecular phylogeny analyses; Marasek-Ciolakowska et al., 2018). Most wild species belonging to the Sinomartagon/Daurolirion section accumulate carotenoids, including paprika color carotenoid capsanthin, in flower tepals (Wang and Yamagishi, 2019; Yamagishi et al., 2010a). Plants that accumulate capsanthin in flowers are rare (Ohmiya et al., 2019). In addition to carotenoids, some wild species, including L. dauricum, L. bulbiferum, and L. cernuum, accumulate anthocyanins in their tepals (Yamagishi and Nakatsuka, 2017; Yamagishi et al., 2014a). Tepals of L. dauricum and L. bulbiferum accumulate both carotenoids and anthocyanins, and thus exhibit red coloration (Yamagishi and Nakatsuka, 2017), while those of L. cernuum accumulate anthocyanins only, producing pink flowers (Yamagishi, 2020a). Oriental hybrid lilies are derived from crosses among species of the Archelirion section (Yamagishi et al., 2014a). Wild species belonging to the Archelirion section often accumulate anthocyanins, but rarely accumulate carotenoids in their tepals. Thus, the major visible pigments in Oriental hybrid lily flowers are anthocyanins (Yamagishi et al., 2014a).
The genetic background of flower anthocyanin pigmentation has been well evaluated in lilies (Sakai et al., 2019; Suzuki et al., 2016; Yamagishi, 2013). The Lilium genome contains several subgroup 6 members of R2R3-MYB genes that positively regulate anthocyanin biosynthesis. Anthocyanin biosynthesis in tepals is primarily regulated by MYB12 in Asiatic and Oriental hybrid lilies; if lily plants carry the MYB12 gene, entire tepal regions are usually anthocyanin-pigmented (Lai et al., 2012; Yamagishi, 2011; Yamagishi et al., 2010b, 2012). Anthocyanin color patterns are often found in the flowers of wild lilies and lily cultivars, which are primarily caused by the spatially restricted expression of R2R3-MYB positive regulators, including MYB15, MYB18, MYBSPLATTER, MYB19L, and MYB19S (Yamagishi, 2016, 2018, 2020c; Yamagishi et al., 2014b). The most typical color pattern is raised spots, where the interior surfaces of tepals increase to develop bumps, and anthocyanin pigments accumulate at the bumps (Yamagishi and Akagi, 2013). MYB19L and MYB19S are responsible for anthocyanin biosynthesis in the bumps of Asiatic hybrid lilies (Yamagishi, 2020b), while MYB12 regulates anthocyanin accumulation at both raised spots and whole tepals in Oriental hybrid lilies (Yamagishi, 2011, 2021).
The miR828/MYB12 module is responsible for the creation of bicolor tepals with white lower halves and colored upper halves, and such bicolor tepals are found among Asiatic hybrid lily cultivars (Yamagishi and Sakai, 2020), but not in other hybrid groups of lilies. However, from which wild species the bicolor tepal trait is derived remains to be clarified. Lilium dauricum (the Daurolirion section) is a parental wild species used to breed Asiatic hybrid lilies. As MYB12 nucleotide sequences in L. dauricum are the same as or similar to those isolated in Asiatic hybrid lily cultivars, L. dauricum likely donated the MYB12 gene to the Asiatic hybrid lily cultivars (Yamagishi and Nakatsuka, 2017). Flowers of L. dauricum, which accumulate both anthocyanins and carotenoids, often show red upper halves and orange–yellow lower halves in single tepals (Fig. S1). Thus, a spatially different pigment deposition pattern has been hypothesized.
The common type of L. cernuum has pink flowers, whereas L. cernuum var. album has white tepals with partially pink regions. The proportion of colored areas in L. cernuum var. album tepals varies among plants, as some plants show pink coloration around the midribs of tepals and some plants exhibit pink-colored regions only at tepal tips (Fig. S1). We previously clarified that MYB12 predominantly regulates anthocyanin biosynthesis in L. cernuum, but MYB12 expression is severely suppressed in L. cernuum var. album, resulting in white tepal creation (Yamagishi, 2020a). The Oriental hybrid lily ‘Dizzy’ exhibits red pigmentation around the midribs of tepals with white marginal regions. Many raised spots are also found in the tepals (Fig. S1). Anthocyanins accumulate in the red central region around the midribs. Transcriptome analysis of ‘Dizzy’ tepals indicates that transcript accumulation of the MYB12 gene, as well as anthocyanin biosynthesis genes, is higher at red central regions than in white marginal regions, indicating that spatially distinct MYB12 expression primarily causes the bicolor pattern in ‘Dizzy’ flowers (Yamagishi et al., 2018). In both lilies, the suppression of MYB12 expression caused the white regions of tepals. However, the mechanisms by which MYB12 expression is suppressed in var. album and in ‘Dizzy’ is unclear.
Subgroup 6 members of R2R3-MYB genes in Lilium species have miR828 target sequences. Similarly, subgroup 6 members of R2R3-MYB genes in some eudicot species, including Arabidopsis thaliana and Salvia miltiorrhiza, and many monocot species contain miR828 target sequences; therefore, these are potentially regulated by miR828 directly (Yamagishi and Sakai, 2020). Bicolor pigmentation is found in flowers of Salvia microphylla (cherry sage), Tulipa gesneriana, and Alstroemeria sp. (Fig. S2), although whether spatially distinct accumulation of miR828 in single petals/tepals causes the bicolor pigmentation is not known in these species.
The purpose of this study was to clarify whether the module consisting of miR828 and subgroup 6 members of R2R3-MYB generally participates in color pattern development in flowers of lilies and other plants. Firstly, the involvement of the miR828/MYB12 module in the spatially different pigment deposition found in L. dauricum flowers was analyzed. Secondly, whether miR828 downregulated MYB12 expression was examined in flowers of L. cernuum var. album and Oriental hybrid lily ‘Dizzy’. Finally, miR828 accumulation levels were compared between the red and white regions of bicolor flowers in cherry sage, tulip, and Alstroemeria.
Bulbs of two tulip cultivars ‘Match’ and ‘Symphony’ (Tulipa gesneriana), and of an Oriental hybrid lily cultivar ‘Dizzy’ (Lilium sp.), and potted plants of Alstroemeria Colorita® Katiana (Alstroemeria sp.) and cherry sage (Salvia microphylla) were purchased at a local market. Lilium dauricum, L. cernuum, and L. cernuum var. album were propagated by seed. All plants were grown in a greenhouse (unheated and natural photoperiod) at the experimental farm at Hokkaido University, Sapporo, Japan. Flower buds were harvested one (in most cases) or two days before anthesis. Two L. dauricum, L. cernuum, and L. cernuum var. album plants were used to evaluate microRNA accumulation and gene expression because the genetic background of these wild species varied among plants.
Pigmentation in L. dauricum tepals was analyzed as follows. Surfaces of the outer tepals were cut up using a knife to enhance the pigment extraction and then whole outer tepals were soaked in 5% formic acid solution for three days to remove anthocyanin pigments.
Low molecular weight RNA (LMW RNA) and normal molecular weight RNA (total RNA) were separately isolated from petals (cherry sage) or inner tepals (other plant materials) using a High Pure miRNA Isolation Kit (Roche Diagnostics K. K., Tokyo, Japan). To evaluate mature miR828 accumulation, stem-loop pulsed reverse transcription from LMW RNA and end-point PCR (in tulips, cherry sage, and Alstroemeria) or quantitative end-point PCR (in Lilium species) were performed following the protocol described by Varkonyi-Gasic et al. (2007). U6 small nuclear RNA was reverse transcribed from the LMW RNA with the U6br primer and subsequently amplified by PCR under the same conditions as the end-point PCR to normalize the accumulation levels of miR828. A PrimeScript™ II 1st strand cDNA Synthesis Kit (Takara Bio Inc., Shiga, Japan) was used to synthesize miR828 and U6 cDNA.
To estimate the expression levels of MYB12, dihydroflavonol 4-reductase (DFR), and anthocyanidin synthase (ANS), cDNA was synthesized from total RNA using the ReverTraAce® qPCR RT Master Mix with gDNA Remover (Toyobo Co., Ltd., Tokyo, Japan) and used for quantitative PCR (40 cycles of [94°C 20 s, 55°C 30 s, and 72°C 20 s]). Lily ACTIN was used to normalize the mRNA accumulation. The primers used in this study are listed in Table S1.
Quantitative PCR was conducted using THUNDERBIRD® SYBR® qPCR Mix (Toyobo), and signals were monitored using the CFX Connect Real-Time System (Bio-Rad Laboratories Inc., Tokyo, Japan). MiR828 accumulation levels and mRNA expression levels were estimated using the formula: 2−ΔCt, where ΔCt = Ct(target gene) − Ct(U6 or ACTIN). Three flowers per plant were used as replicates to calculate the mean values and standard errors. Statistical differences were analyzed using t-tests.
Tepals of L. dauricum exhibited red upper halves and orange–yellow lower halves, in addition to many dark red raised spots (Fig. 1A, C). As upper tepal parts, excluding raised spots, accumulated anthocyanin pigments (cyanidin 3-rutinoside, Yamagishi and Nakatsuka, 2017), it was expected that the upper halves accumulated both anthocyanins and carotenoids (thus producing a red color), while the lower half regions mainly accumulated carotenoids, but contained small amounts of anthocyanins other than at the raised spots. To confirm this hypothesis, anthocyanin pigments were removed by soaking the whole tepals in 5% formic acid solution (Fig. 1B, D). Then, the color at the upper halves turned from red to orange–yellow, and the color difference between the upper and lower halves became smaller, indicating that higher anthocyanin accumulation in the upper halves than in the lower halves causes bicolor patterns. This spatially different anthocyanin deposition pattern was similar to that found in the bicolor tepals of Asiatic hybrid lilies. After soaking, whole tepal regions were orange–yellow, possibly due to the accumulation of carotenoids, including the paprika color carotenoid capsanthin (Wang and Yamagishi, 2019; Yamagishi et al., 2010a). The dark red coloration at the raised spots also disappeared after soaking because the major pigments in these areas are anthocyanins.
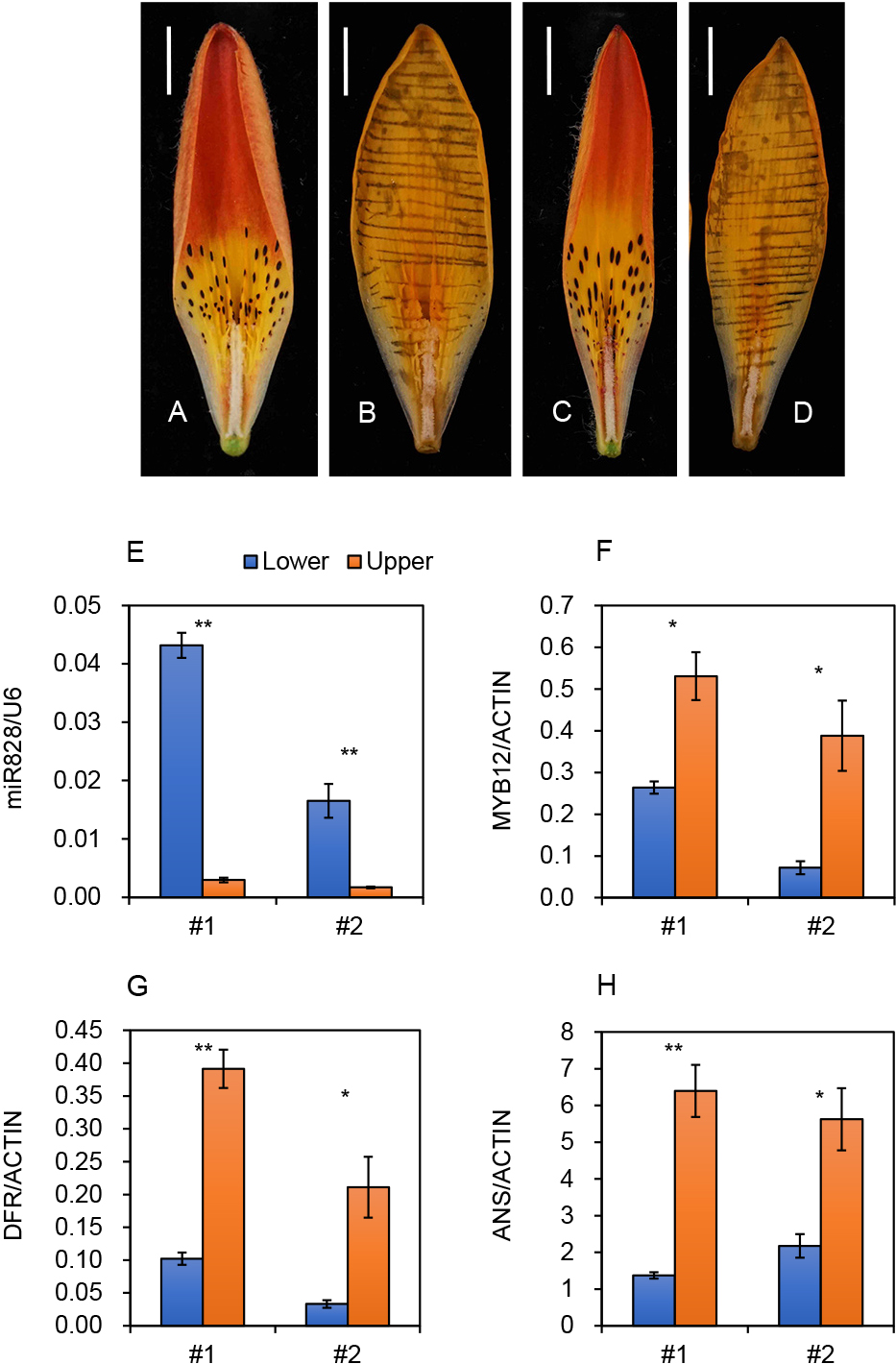
Color appearance (A–D) and accumulation of mature miR828 and mRNA transcripts (E–H) in tepals of L. dauricum (one day before anthesis). A, C: Outer tepals. B, D: Outer tepals after removing anthocyanin pigments by soaking them in 5% formic acid solution. Tepals in A and C were derived from different plants. White bar = 1 cm. E–H: Accumulation of miR828 (E) and expression of MYB12 (F), DFR (G), and ANS (H) in lower and upper tepal regions of two plants (#1 and #2). Accumulation was normalized against U6 RNA or ACTIN mRNA. Vertical bars show standard error (SE) of the means of three flowers. * and ** indicate significant difference at 5 and 1% levels, respectively (t-test).
The involvement of miR828 accumulation in spatially different anthocyanin coloration was examined using L. dauricum tepals (Fig. 1E). Two plants were used because the genetic backgrounds of wild species varies among plants. MiR828 accumulation levels were more than 10 times higher in the lower halves than in the upper halves of both plants, indicating high miR828 accumulation in the tepal regions with poor anthocyanin pigmentation. To confirm that miR828 specifically accumulated at the lower halves, accumulation levels of other microRNAs were evaluated using the same LMW RNA. MiR156 influences anthocyanin accumulation in vegetative organs of Arabidopsis (Gou et al., 2011). MiR156 accumulation levels were similar between the lower and upper tepal halves in plant #1, and that in the lower halves was higher than that in the upper halves in plant #2, but less than two-fold that of the upper halves (Fig. S3). Thus, miR156 accumulation did not show much difference between the pigmented and un-pigmented regions in L. dauricum tepals.
MYB12 is a direct target of miR828 (Yamagishi and Sakai, 2020). Transcript accumulation of MYB12 was higher in the upper tepal halves than in the lower tepal halves (Fig. 1F), that is, miR828 accumulation and MYB12 expression exhibited a negative correlation with each other between the pigmented and un-pigmented regions. Expression of two anthocyanin biosynthesis genes, DFR and ANS, was significantly higher in the upper halves than in the lower halves (Fig. 1G, H), indicating that their expression also showed a negative correlation with miR828 accumulation.
MicroRNA828 accumulation in L. cernuum flowersThe involvement of the miR828/MYB12 module in flower pigmentation was evaluated in other lilies. L. cernuum flowers accumulated anthocyanins and had pink coloration. Lilium cernuum var. album had white tepals, but regions at tepal tips or around midribs were anthocyanin-pigmented, although color intensity in colored regions was weaker than that in L. cernuum tepals (Fig. 2). As suppression of MYB12 transcription factor gene expression is likely the primary cause of low anthocyanin accumulation in L. cernuum var. album (Yamagishi, 2020a), miR828 involvement in MYB12 suppression was evaluated by comparing miR828 accumulation levels between L. cernuum and L. cernuum var. album. If miR828 directly suppresses MYB12 expression, miR828 accumulation levels should be high in L. cernuum var. album. Experiments were performed twice using different plants because L. cernuum is a wild plant and its genetic background is not uniform (Fig. 2). In the first experiment, miR828 levels were higher in L. cernuum var. album compared to L. cernuum, but less than two-fold that of L. cernuum. In the second experiment, miR828 levels were higher in L. cernuum than in L. cernuum var. album. In both experiments, miR828 accumulation levels were not high, similar to those detected in the upper halves of L. dauricum tepals. Thus, we concluded that the differences in miR828 accumulation levels between L. cernuum and L. cernuum var. album were unlikely to be involved in the suppression of MYB12 expression.

Color appearance and miR828 accumulation in tepals of Lilium cernuum var. album (L.c. album) and L. cernuum. Upper: Color appearance of flowers. Lilium cernuum var. album had white coloration with partially pink tepals. White bar = 1 cm. Lower: MiR828 accumulation in tepals of L. cernuum var. album and L. cernuum. Different plants were used for the first and second experiments. Accumulation was normalized against U6 RNA. Vertical bars show SE of the means of three flowers. * indicates significant difference at 5% levels (t-test).
Tepals of an Oriental hybrid lily ‘Dizzy’ exhibited red-pigmented regions around the midribs and white marginal regions (Fig. 3A). As MYB12 expression is high in the regions around the midribs, but only trace levels are detected at the marginal regions (Yamagishi et al., 2018), the involvement of miR828 accumulation in spatially distinct MYB12 expression was examined using ‘Dizzy’ tepals collected one day before anthesis. Compared with the regions around the midribs, the miR828 accumulation level was very low in the white marginal regions (Fig. 3B), indicating that miR828 does not suppress MYB12 expression directly at the marginal regions.

A: Color appearance of an Oriental hybrid lily ‘Dizzy’ one day before anthesis. White bar = 1 cm. B: MiR828 accumulation at white marginal (white) and red central (red) regions of ‘Dizzy’ tepals. Accumulation was normalized against U6 RNA. Vertical bars show SE of the means of three flowers. ** indicates significant difference at 1% levels (t-test).
To determine whether the module consisting of miR828 and subgroup 6 members of R2R3-MYB was involved in pattern development in species other than Lilium, four materials (three species) with bicolor flowers were evaluated (Fig. S2). In bicolor tulips ‘Match’ and ‘Symphony’, tepal tips were anthocyanin-pigmented one or two days before anthesis, and these pigmented areas expanded at anthesis. Pigmented areas were wider in ‘Symphony’ than in ‘Match’. Of the petals in the cherry sage, the upper regions were red, while the basal regions were white. Alstroemeria ‘Katiana’ has three inner tepals, and two of the three showed bicolor pigmentation at anthesis. However, all three inner tepals showed similar color appearance one day before anthesis: a top pigmented region and a wider white region.
Petals of cherry sage and inner tepals of tulip and Alstroemeria were collected one or two days before anthesis and were separated into colored and white parts. Next, miR828 accumulation levels were compared between the parts (Fig. 4). In tulip, miR828 accumulation levels were relatively low in both the white and pink parts compared to those in other species, and the difference in the levels was small between the two parts. In cherry sage and Alstroemeria, miR828 levels were similar between the white and pink parts or higher in the red part than in the white part. If miR828 directly suppresses the action of R2R3-MYB positive regulators, miR828 accumulation should be high in the unpigmented region. Thus, these results suggest that the module of miR828 and R2R3-MYB positive regulators is unlikely to be involved in bicolor flower development in these plants.

miR828 accumulation in white (W) and red (R) petal/tepal regions of tulip (two cultivars), cherry sage, and Alstroemeria. miR828 and U6 were amplified from the same low molecular weight RNA. Numerals in parentheses indicate the PCR cycle number.
MiR828 is a highly conserved microRNA identified in a wide range of plant species. Sequences of mature miR828 are identical in many species (Yamagishi and Sakai, 2020), while that in Arabidopsis is one nucleotide different (Rajagopalan et al., 2006). MiR828 often suppresses the expression of R2R3-MYB genes belonging to subgroups 15 and 4, which negatively regulate anthocyanin biosynthesis (Xia et al., 2012; Zhu et al., 2012). Therefore, the amount of anthocyanin pigments increases in grape berries and potato tubers with an increase in miR828 accumulation (Bonar et al., 2018; Tirumalai et al., 2019). In Arabidopsis, miR828 directly and indirectly targets AtMYB113 and AtMYB75 (AtPAP1), which are subgroup 6 members of R2R3-MYB genes, positively regulating anthocyanin biosynthesis in vegetative organs, and the amount of anthocyanin pigments decreases with an increase in miR828 accumulation (Rajagopalan et al., 2006). MiR828 in Arabidopsis is involved in the negative feedback regulation of anthocyanin accumulation under stress conditions (Luo et al., 2012). In grapes, potato, and Arabidopsis, miR828 acts in fruits, tubers, and vegetative organs, but not in flowers, and does not create flower color patterns. Thus, miR828 mediating bicolor pattern formation is known only in Asiatic hybrid lily flowers (Yamagishi and Sakai, 2020). In this study, we further examined whether the module of miR828 and subgroup 6 members of R2R3-MYBs was involved in other lilies and in other species.
Lilium dauricum exhibited bicolor tepals, of which the upper halves accumulated anthocyanins, but the lower halves contained low levels of anthocyanins. The profiles of miR828 accumulation and MYB12 expression in tepals, higher accumulation of miR828, and lower expression of MYB12 at the lower halves than at the upper halves, were similar to those found in bicolor cultivars of Asiatic hybrid lilies. That is, the bicolor flower pattern was developed by the same mechanism in L. dauricum and in Asiatic hybrid lily cultivars. Given that L. dauricum is among the parental wild species for Asiatic hybrid lily establishment (Yamagishi and Nakatsuka, 2017), it is possible that the bicolor tepal trait in Asiatic hybrid lilies is derived from L. dauricum.
Although miR828 accumulation and MYB12 expression exhibited a negative correlation with each other, MYB12 expression was detected to some extent in unpigmented lower regions, where miR828 accumulation levels were high. Similar results have been reported in the bicolor tepals of Asiatic hybrid lilies (Yamagishi and Sakai, 2020). MicroRNA not only cleaves target gene transcripts, but also arrests their translation (Dalmay, 2013; Gandikota et al., 2007; Gattolin et al., 2018; Wang et al., 2016). MiR828 is also likely to undergo both transcript cleavage and translation suppression, so not all MYB12 transcripts are cleaved. Thus, these results strongly suggest that miR828 directly arrests the action of MYB12 preferentially at the lower halves of L. dauricum tepals. Expression of DFR and ANS genes was also suppressed at the unpigmented regions. However, compared to the bicolor tepal cultivars of Asiatic hybrid lilies, their expression levels in the lower halves were relatively high in this study. In previous reports, the expression levels of anthocyanin biosynthesis genes were very low in the lower halves of bicolor tepals (Suzuki et al., 2016; Yamagishi and Sakai, 2020). While the lower halves included raised spots in this study, raised spots were not included in previous studies, which may have caused this discrepancy. Expression of anthocyanin biosynthesis genes at raised spots was regulated by MYB19S and MYB19L and peaked earlier than that at tepal regions other than the spot regions, but their expression was detected to some extent at one day before anthesis (Yamagishi, 2020b) when tepals were collected in this study.
The involvement of miR828 in the post-transcriptional regulation of MYB12 expression was evaluated in other lilies. The miR828/MYB12 module is unlikely to be responsible for the color patterns found in the Oriental hybrid lily ‘Dizzy’. That is, the module consisting of miR828 and R2R3-MYB positive regulators is responsible only for bicolor patterns in upper pigmented and lower unpigmented halves. In ‘Dizzy’, miR828 accumulation was relatively high at pigmented central regions, suggesting miR828 involvement in negative feedback regulation of anthocyanin accumulation, as shown in Arabidopsis (Luo et al., 2012).
In the stems of Arabidopsis, anthocyanins accumulate in an acropetal manner, that is, higher in basal parts and lower in apical parts. Arabidopsis SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 9 (SPL9) prevents anthocyanin accumulation by directly preventing the expression of anthocyanin biosynthesis genes. MiR156 accumulates more highly in the basal parts than in the apical parts and downregulates SPL9 expression post-transcriptionally, thereby causing anthocyanin accumulation in the basal parts (Gou et al., 2011). Accumulation of miR828 in lilies and miR156 in Arabidopsis in an acropetal manner creates color patterns, although the microRNA families and targets involved are different. Thus, the next experimental theme will be to clarify the mechanisms by which miR828 accumulates in an acropetal manner.
In tepals of L. cernuum var. album, regions at tepal tips or around midribs were anthocyanin-pigmented, although color intensity in colored regions was weaker than that in L. cernuum tepals, indicating that expression of MYB12 is likely suppressed in the entire tepal region (Yamagishi, 2020a). In this study, miR828 accumulation levels were not high in tepals either of L. cernuum or L. cernuum var. album, so miR828 is unlikely to be involved in the expression suppression of MYB12. Weak MYB12 expression in L. cernuum var. album is caused by another mechanism, which includes mutation at the promoter region (changes in cis-regulatory elements) and the presence or absence of a transcription factor. Cis-regulatory elements and transcription factors that suppress or enhance the expression of subgroup 6 members of R2R3-MYB genes have been identified in fruits and vegetative organs (Fang et al., 2019; Gangappa and Botto, 2016), but the knowledge about such factors is limited in flowers (Jiang and Rausher, 2018).
In tulip, cherry sage, and Alstroemeria, high miR828 accumulation was not detected at uncolored areas of bicolor petals/tepals. Although R2R3-MYBs positively regulating anthocyanin biosynthesis are not characterized in these species and whether they include miR828-recognition sites is unknown, our results suggest that the mechanisms by which bicolor patterns developed in these species were likely different from those that create bicolor tepals in Asiatic hybrid lilies and L. dauricum. This observation suggests that miR828/R2R3-MYB module-involved pattern development is likely specific to Lilium species.
In conclusion, the module of miR828 and R2R3-MYB positive regulators is involved only in bicolor development of lily flowers with upper pigmented and lower un-pigmented tepals, and is unlikely to be involved in color pattern development in other species. Lilium dauricum is a candidate donor that has donated the bicolor trait to Asiatic hybrid lilies.