2014 Volume 54 Issue 2 Pages 374-383
2014 Volume 54 Issue 2 Pages 374-383
Carbide precipitation and eutectic phase transformation during solidification of Fe–C–Cr–Ti–Mn–Mo–Ni–Si Ti–added high-chromium cast irons (HCCIs) were studied numerically and experimentally by the help of Partial Equilibrium approximation, DSC thermal analyses and EDX analyses. The main carbides formed during the solidification are distinguished as MC, primary M7C3 and eutectic M7C3 from their distinguished constitution, while other researchers didn’t distinguish the primary and eutectic M7C3 carbide. Through comparing the prediction of Partial Equilibrium approximation with DSC thermal analysis measurement, the precipitation sequence of the eutectic structure in HCCIs is clarified to follow the sequence of FCC prior to the eutectic M7C3, although they were usually expected to precipitate simultaneously. The hardness index of the HCCIs is evaluated quantatively by summation of the contributions of the Vickers hardness of MC, primary M7C3 and eutectic M7C3 carbides with predicted precipitation amount and composition / constitution. The effects of C, Ti and Cr contents on the precipitation sequence, the amount and the composition of carbides as well as the hardness of the HCCIs are discussed deeply. Finally, the validity of Partial Equilibrium approximation is shown in prediction of the solidification in multicomponent system with large amount of precipitated carbides.
High Chromium Cast Irons (HCCIs) have been well-known as wear-resistant materials with the high-hardness M7C3 carbide. With the request to improve their wear-resistance, much amounts of carbides are expected, resulting in the application of hypereutectic HCCIs in which M7C3 precipitates as primary as well as eutectic carbide, instead of hypoeutectic ones. At the same time, in order to refine the coarse primary M7C3 carbide, some techniques such as alloying are adopted in solidification process. Among the added elements, Titanium is chosen for not only refining the grain size of M7C3 carbide but also forming TiC carbide with even higher hardness itself.
Our previous study by Liu, et al.,1) and other studies by Zhi, et al.,2) Chung, et al.3) and Bedolla-Jacuinde, et al.4) have reported the experimental results on Fe-4wt%C-17wt%Cr, Fe-4wt%C-20wt%Cr, Fe-4wt%C-25wt%Cr and Fe-2.5wt%C-16wt%Cr high chromium cast irons with different Ti-additions, as shown in Table 1. They found that, besides the refinement of M7C3 carbides, the volume fraction of M7C3 carbide also decreases with Ti addition, as shown in Fig. 1. However, the effects of Ti and other constituents of HCCI on the precipitation sequence, the amount and the composition of carbides were not discussed in detail.

Volume fraction of M7C3 and TiC carbides for alloy L1–L4 using PE approximation. “·” denotes the transformation point from primary M7C3 to eutectic M7C3.
On the other hand, in the case of small amount of precipitated carbides, the validity of Partial Equlibrium (PE) approximation which considers the back-diffusion of carbon in solids such as FCC and BCC but neglects it in carbides has been proved on the application to Fe–C–Cr alloys by Chen and Sundman5) and Fe–C–V–W–Cr–Mo high speed steels in our previous study by Zhang, et al.6,7)
In the present paper, the validity of Partial Equilibrium (PE) approximation5,6,7) in the case of large amount of precipitated carbides is discussed through applying to Fe–C–Cr–Ti–Mn–Mo–Ni–Si Ti-added HCCIs. First, the carbide precipitation mechanism during solidification is carefully analyzed on Fe-4wt%C-17wt%Cr-1.5wt%Ti based alloys. At that time, the predicted precipitation sequence is compared with those in DSC thermal analyses and the predicted micro-segregation in each phase is compared with the measured composition of M7C3 carbide using the experimental samples made in our previous study by Liu, et al.1) The composition variation in primary and eutectic M7C3 carbide can be distinguished both in prediction and measurement. It is helpful to make clear the effects of carbide composition variation on its hardness. Then, for all the alloys in Table 1, the effects of carbon, titanium and chromium on the precipitation sequence, the amount and the composition of carbides are discussed in details with the help of previous experimental results,1,2,3,4) from the viewpoint of hardness control by carbides. Finally, it is confirmed that PE approximation clearly presents its versatile application up to the case of large amount of precipitated carbides.
The solidification experiments on Fe–C–Cr–Ti–Mn–Mo–Ni–Si Ti-added HCCIs (four alloys: L1 to L4) were performed in our previous study by Liu. et al.1) The raw materials were melted inside a medium frequency induction furnace (45 kW, 7 kHz) with Ar gas atmosphere. Then, the molten alloy was poured into three kinds of moulds (metal, silica sand and graphite, respectively) with 120 mm length × 84 mm width × 55 mm height. The thermocouples inserted into the protective tube were positioned in the middle-width section of the mould at site 1 (15 mm to right sidewall and 22 mm to bottom) and site 2 (5 mm to bottom and 60 mm to right sidewall) to record temperature histories, as shown in Fig. 2(a). Unfortunately, the temperature curves obtained from this method as seen in Fig. 2(b) could only give the rough estimation of eutectic temperature and the temperature of solidification end. As a result, those curves were useless to validate PE approximation due to the sensitivity limitation.

a) Schematic ingot casting process and temperature measuring positions and b) Typical cooling curves measured for hypereutectic HCCIs with 1.5 wt% Ti addition in cooling rate –0.40 K/s (–24.0 K/min) in sand mold, –1.34 K/s (–80.2 K/min) in graphite mold and –1.78 K/s (–107.0 K/min) in metal mold, respectively. The dot-dash curve denotes the numerical fitting of cooling rate. (Online version in color.)
In order to analyze each phase precipitation temperature of the above four alloys: L1 to L4, the solidification behavior is examined carefully by Differential Scanning Calorimetry (DSC) thermal analysis using the same DSC apparatus in our previous studies by Nakajima, et al.8,9,10) The samples were cut from the zone near site 1. The chemical compositions for the present samples, alloy L1 to L5, are shown in Table 1, in which the compositions of other researchers’ samples: alloy Z1 to Z4, C1 to C4 and B1 to B5 are also shown. A small piece of sample was machined into a disc (4.3 mm in diameter, 2 mm in height) and melted then solidified in an alumina crucible under an ultra-purity Ar gas atmosphere in DSC apparatus. The heating rate was kept constant at 20 K/min up to 1600°C. Then, the precipitations of each solid phase were measured at the cooling rate of –5, –20 and –70 K/min, respectively. The precipitation temperatures of solid phases were estimated through the DSC curve. Here, the solidus temperature in heating process or the temperature of solidification end in cooling process, Tsol , was defined as the tangential intersection of the baseline and the line from the heat peak on DSC curve, as shown in Fig. 3.

DSC curves for Alloy L3 (Ti = 1.5 wt%) at cooling rate (a) –5 K/min (b) –20 K/min and (c) –70 K/min with the same heating rate 20 K/min. Eu1 and Eu2 denote the eutectic heat release peaks in DSC cooling process. (Online version in color.)
The precipitation temperatures of primary M7C3 and eutectic structure M7C3 + FCC and the solidus temperature, Tsol, during heating process were reproduced, within the difference of 0 to 21°C, 16 to 24°C and 15 to 31°C respectively, in accordance with the pseudo binary equilibrium phase diagram calculated using Thermo-Calc, as shown in Fig. 4. It is judged, from these results, that the present DSC thermal analysis is reliable.

Comparison of the phase precipitations in DSC heating process with pseudo binary phase diagrams of High Cr cast irons. The effects of (a) C, (b) Ti and (c) Cr content are also shown.
The samples of alloy L1–L4 cut at the position site 1 in graphite-mold solidification were used for determination of M7C3 carbide constitution The compositions of primary and eutectic M7C3 at the room temperature were measured by Energy Dispersive X-ray spectrometer (EDX). Figure 5 shows the measured compositions for alloys L1 to L4. Here, 11.2 μm was adopted as the critical size between primary and eutectic M7C3 carbides, according to our previous study by Liu. et al.1)
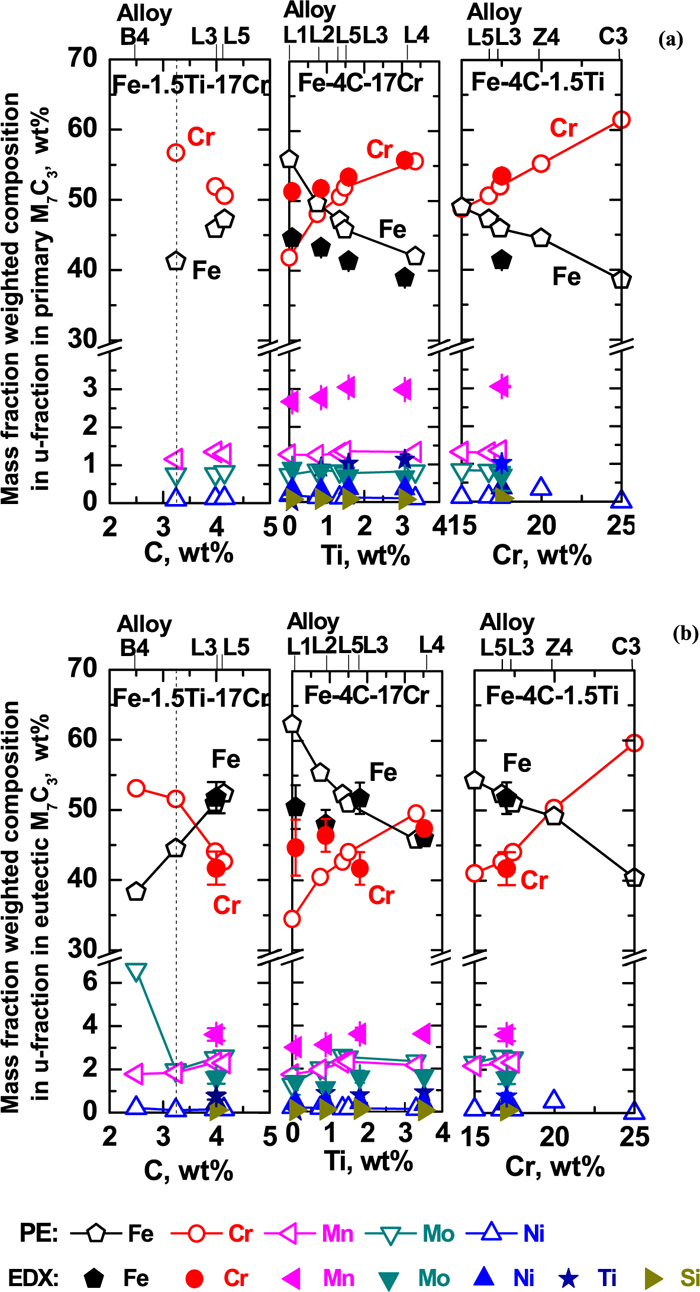
Effects of C, Ti and Cr contents on (a) primary and (b) eutectic M7C3 carbide compositions. The samples of alloy L1–L4 were cut at the position site 1 in graphite-mold solidification. The measured M7C3 compositions by EDX for alloy L1–L4 are labeled as solid symbols. Correspondingly the predicted compositions by PE approximation for alloy L1–L5, B4, Z4 and C3 are denoted as solid lines with open symbols. The dotted vertical line denotes the transition point from hypoeutectic to hypereutectic in the pseudo phase diagram in Fig. 4(a).
Solidification path of Fe–C–Cr–Ti–Mn–Mo–Ni–Si Ti-added HCCIs can be predicted numerically by coupling the Partial Equilibrium (PE) approximation with thermodynamic equilibrium calculations. The PE approximation, based on the ideas of Chen and Sundman,5) assumes negligible diffusion in solids for substitutional elements such as iron and chromium et al. and complete diffusion in solids for interstitial elements such as carbon in the present HCCI. Except the stoichiometric and semi-stoichiometric phases e.g. carbides in which the compositions of interstitial elements are fixed, the existing phases containing interstitial element like carbon always take part in partial equilibrium calculation.
The partial equilibrium sequence obeys the following equations:
• Equal chemical potential of carbon between liquid, l and a solid, s1 :
| (1) |
• Mass conservation of carbon during partial equilibrium:
| (2) |
• Unchanged u-fraction of substitutional elements in each phase during partial equilibrium:
| (3) |
• Mass conservation of substitutional elements during partial equilibrium:
| (4) |
The partial equilibrium among several phases (i.e. liquid and solid phases sj, j = 1, 2, …, m) is assumed equivalent to several two-phase partial equilibriums6,7) (e.g. liquid and solid s1, liquid and solid s2, … , liquid and solid sm). Thus the mass conservation of carbon among all phases follows:
| (5) |
Detailed explanation of the above equations can be found in our previous study by Zhang et al.6,7) The program of the Partial Equilibrium (PE) approximation for Fe–C–Cr–Ti–Mn–Mo–Ni–Si Ti-added HCCIs is performed using the TQ program interface for coupling thermodynamic equilibrium calculations made by Thermo-Calc11) and access to the thermodynamic properties of steels from database TCFE6.12) The chemical potential of carbon in a phase is determined through phase equilibrium calculation providing a phase composition, temperature and pressure. The temperature step adopted in the calculations is –1 K. The calculations finish at the mass solid fraction greater than 0.99.
The carbide precipitation mechanism during solidification is carefully analyzed for alloys L1 to L5. At that time, the prediction of precipitation sequence and consequent micro-segregation in each phase is compared with those in DSC thermal analyses and M7C3 carbides’ composition measurements.
4.1.1. Phase Precipitation SequenceDue to the solute redistribution at solid/liquid interface, the composition in residual liquid evolves with the precipitation of solid phases. For example, as shown in Fig. 6, for Fe-4wt%C-17wt%Cr-1.5wt%Ti alloy, that is the nominal or average composition of alloy L3, the precipitation of solids follows the order of MC (1576°C) → primary M7C3 (1329°C) → eutectic M7C3 + FCC (1256°C) → M3C (1109°C). Corresponding to these precipitations, the composition in residual liquid decreases in the order of Ti → Cr → Fe. MC carbide precipitates firstly (with decrease of Ti) and acts as the nuclei of the primary M7C3 carbide (with decrease of Cr).1) Afterwards forms the eutectic M7C3 + FCC as the matrix of HCCI. M3C carbide forms at the final stage of solidification, which results in the decrease of Fe and the increase of Mo and Mn in residual liquid. Since M3C precipitates near the end of solidification, it can be deduced that M3C may be present in only a small amount. From Fig. 6(a), the amount of MC and M7C3 reaches over 33% in volume fraction, thus Fe-4wt%C-17wt%Cr-1.5wt%Ti alloy is recognized as HCCI with large amount of precipitated carbides. On the other hand, as shown for alloy L3 in Figs. 3(a) and 7, the same order of MC (no detection) → primary M7C3 (1322°C) → eutectic M7C3 (or FCC)(1247°C) → FCC (or eutectic M7C3)(1217°C) → M3C (no detection) was detected from DSC thermal analysis on cooling process. Although unfortunately MC and M3C could not be detected due to the small precipitation amount, eutectic M7C3 and FCC which forms as the result of eutectic transformation were separately detected.

Predicted (a) solidification path and (b) composition evolution in liquid for the average composition of alloy L1 to L5 by PE approximation. (Online version in color.)

Demonstration of the eutectic transformation process on DSC cooling curve.
Double eutectic heat peaks on DSC cooling curve can be observed. As shown in Fig. 3, there is a single heat absorbing peak relating to the melting of eutectic structure in DSC heating process, while there are double heat release peaks relating to the eutectic transformation in DSC cooling process. The wide interval of the double peaks becomes narrower with the cooling rate being larger (–5 K/min to –70 K/min). The sequent appearance of the eutectic phases is the direct result of diffusion-dominated solidification process, where the phases constituting the eutectic structure M7C3 + FCC appear one by one but not precipitate simultaneously as usually expected. Figure 7 illustrates the possible appearing sequence of eutectic M7C3 and FCC. One possible case is shown in Fig. 7(a), after the formation of primary M7C3, the eutectic M7C3 precipitates firstly (corresponding to the first eutectic peak), so the eutectic transformation starts from the formation of FCC phase (the second eutectic peak), i.e. at 1216.9°C. From the composition analysis in Fig. 5, it is clear that the chemical compositions of primary and eutectic M7C3 are different. It makes possible that the peak denoting the eutectic M7C3 is after the formation of primary M7C3, although the eutectic M7C3 precipitates continuously after the primary M7C3 precipitation. In contrast, the other possible case is as shown in Fig. 7(b), the FCC forms firstly (the first eutectic peak) after the primary M7C3, then precipitates the eutectic M7C3 (the second eutectic peak), thus the eutectic transformation starts at 1247.4°C, i.e. also from the formation of FCC phase.
From the pseudo binary HCCI alloy phase diagram (Fig. 4 in our previous work [1]), it is clarified that the eutectic transformation in multicomponent alloy system is different from that in binary alloy system. That is, in multicomponent alloy system, the eutectic transformation does occur within a temperature range but not under a constant temperature. As illustrated from the DSC curves in Figs. 3 and 7, the eutectic transformation starts from one constituting phase precipitation (1st heat peak) and in a delayed time or an undercooled temperature following the other phase precipitation (2nd heat peak). The gap between two peaks decreases with the faster diffusion of solutes under larger cooling rate solidification condition. Normal eutectic structures of alloy L1 –L4 can be found in Figs. 7 and 12 in Ref. 1), which indicates that they experienced the normal eutectic transformation.
It also should be mentioned that in the case where thermocouple is commonly protected by Al2O3 tube, it is non-sensitive to the sequent precipitation of phases during eutectic transformation, thus the eutectic transformation seems to occur under a constant temperature. However, with more sensitive and accurate DSC thermal analysis, the double peaks corresponding to sequent precipitating phases could be sensed during eutectic transformation in HCCIs.
Figure 8 shows the predicted solidification sequence and the measured DSC data. As seen, the predicted eutectic temperature much approaches to the measured first eutectic peak, that is, the case of Fig. 7(b). It is also indicated that the precipitation sequence of the eutectic structure in HCCIs starts from the precipitation of FCC prior to the eutectic M7C3. The prediction by PE approximation does show the correct solidification nature and predict the effects of alloy content on phase precipitation sequence, as seen in Figs. 1 and 8, which is comparable to the experimental data, although the PE approximation can’t predict the effects of cooling rate on the precipitation temperature of phases, for instance, the phases in the eutectic transformation as shown on DSC curves in Fig. 3.

Comparison of the phase precipitations in DSC cooling process with PE approximations. The effects of (a) C, (b) Ti and (c) Cr content on the solidification path of High Cr cast irons are also shown. The predicted phase precipitation temperature is deduced from the PE approximation. It corresponds to the highest temperature at which it starts to form. The experimental temperatures are measured from DSC thermal analysis in cooling process.
For Fe-4wt%C-17wt%Cr-1.5wt%Ti alloy, i.e. the nominal or average composition of alloy L3, the carbide precipitation mechanism during solidification is carefully analyzed according to the precipitation sequence: MC, primary M7C3, eutectic M7C3 + FCC. Figure 9 shows the partition coefficient for each solid phase during solidification.

Segregation of elements in (a) MC, (b) M7C3, (c) FCC and (d) M3C phase for the average composition of alloy L1 to L5. (Online version in color.)
Solidification starts from the formation of MC carbide. The partition coefficient of Ti in MC,
Average composition of M7C3 can be estimated from its micro-segregation profile. In fact, the carbide composition is directly related to its hardness. Here, M7C3 carbide precipitates as both primary and eutectic carbide in HCCIs. The composition of primary / eutectic M7C3 carbide is weighted by the newly precipitated amount of phase, i.e.
Figure 5 shows these predicted compositions of primary and eutectic M7C3. The constitution of M7C3 for all HCCIs, including alloys L1 to L5, changes from primary (Cr, Fe)7C3 carbide to eutectic (Fe, Cr)7C3 carbide according to the precipitation temepratures. In addition, both in primary and eutectic M7C3, the composition of Cr increases with both Cr and Ti contents but decreases with C content. The Fe in M7C3 varies opposite to the Cr. Even though Ti is not the dominating element in M7C3, its addition does affect the composition of M7C3 through the formation of MC.
The predicted composition by PE approximation fits with the EDX measured composition for both primary and eutectic M7C3 carbides. The predicted M7C3 composition contains 6 elements: Fe, Cr, C, Mn, Mo and Ni; however, the measured composition includes all 8 elements including Ti. In this way, it gives the evidence that MC acts as nucleation site of both primary and eutectic M7C3.
From the prediction of alloys L1–L5, B4, Z4 and C3, it is confirmed that PE approximation provides clearly the useful information, such as precipitation sequence, amount and composition of all carbides.
4.2. Effects of C, Ti and Cr on Carbide PrecipitationThe wear-resistant properties of HCCI depend mainly on the nature of M7C3 carbide. The experimental observation has already verified that MC carbide (TiC) is the nucleus of M7C3 carbide in our previous study by Liu. et al.1) Thus TiC is helpful to refine the grain size of M7C3 carbide due to increasing the nucleation density. Moreover, TiC itself is also helpful to the wear-resistant properties of HCCI. Since Ti, Cr and Fe, besides C, are the main constituents of MC and M7C3 (from Figs. 5 and 9), the effects of C, Cr and Ti content on the carbide precipitation sequence, amount and composition are deeply discussed.
4.2.1. Prediction Accuracy on Phase Precipitation Temperature and SequenceFigure 8 shows the solid phase precipitation sequence as a function of C, Ti and Cr contents in temperature – composition diagrams. The solid lines with open symbols denote the predicted data by PE approximation and the half solid symbols are the DSC thermal analysis data in cooling process (Here, MC could not be detected due to the small precipitation amount). Within the range of C, Ti, Cr content in Fig. 8, the solid phases precipitate in the order of MC, primary M7C3 and eutectic M7C3 + FCC. Only when C is smaller than 3.24 wt%, phases precipitate in the order of MC, primary FCC and eutectic M7C3 + FCC.
The predicted precipitation temperatures of primary M7C3, FCC and eutectic M7C3 are within 7–40°C, 2–48°C and 2–64°C difference from DSC thermal analysis data respectively, while the predicted temperature of solidification end with fs = 0.98 is within 22–116°C difference from DSC thermal analysis data. It is too difficult to estimate accurately the temperatures of solidification end from DSC thermal analysis. So the differences between the prediction and the DSC thermal analysis are within the allowable range of accuracy. Of course, PE approximation itself has some limitations such as neither considering the diffusion of substitutional elements in solid nor considering the diffusion length related to the solidification structure as well as the limitation of the available alloy database.
In a word, it is indicated that the prediction accuracy on phase precipitation temperature and sequence by PE approximation is also reliable for the case of the large amount of precipitated carbides.
Afterwards, the effects of C, Ti and Cr on solid phase precipitation are discussed from the view point of the relationship among precipitation sequence, precipitation amount and averaged composition of carbides.
4.2.2. Effect of CPrecipitation sequence: It is found in pseudo binary phase diagram (Fig. 4(a)), Fig. 8(a) and Table 1 that the precipitation sequence of solid phases is changed with the increase of C. It follows:
Alloy B1 to B5 (C < 3.24 wt%) and C4 (C > 3.24 wt%, Ti > 5.4 wt%):
MC → primary FCC → eutectic M7C3 + FCC
Alloy L1 to L5, Z1 to Z4, C1 to C3 (C > 3.24 wt%, Ti < 5.4 wt%):
MC → primary M7C3 → eutectic M7C3 + FCC
With the increase of C, the precipitation temperature of MC rises. In addition, at C > 3.24 wt%, the primary phase changes from FCC to M7C3. The precipitation temperature of primary FCC gradually decreases and approaches to the precipitation line of M7C3 + FCC when C < 3.24 wt%, while the precipitation temperature of primary M7C3 gradually increases and separates from that of M7C3 + FCC at C > 3.24 wt%.
Precipitation amount: From Fig. 5, MC(TiC), primary M7C3((Cr, Fe)7C3) and eutectic M7C3( (Fe, Cr)7C3 when C > 3.6 wt%, otherwise (Cr, Fe)7C3 ) are the main carbides. In Fig. 10, the experimental data1,2,3,4) are plotted as solid squares for total M7C3 (primary M7C3 + eutectic M7C3), solid down-triangles for primary M7C3 and solid up-triangles for eutectic M7C3, solid stars for total carbide (total M7C3 + MC) and solid circles for MC, respectively. The predicted data by PE approximation are drawn as solid lines with corresponding open symbols.

As shown in Fig. 10(a), when C < 3.24 wt%, the eutectic M7C3((Cr, Fe)7C3) increase with C content; while when C > 3.24 wt%, the amount of primary M7C3((Cr, Fe)7C3) increases much faster than the decrease of eutectic M7C3((Fe, Cr)7C3), although the amount of MC(TiC) is almost constant. Consequently, the total amount of carbides shows about 15% increase by the increase of C from 2.5 to 4.0 wt%, which is mainly due to the contribution of the increase of primary M7C3((Cr, Fe)7C3).
Composition: Considering the hardness control by carbides, Fig. 5 shows the predicted compositions for primary M7C3 (mainly (Cr, Fe)7C3 ) and eutectic M7C3 (mainly (Fe, Cr)7C3 ) for alloys L1 to L5, B4 and Z4. It is found that with the increase of C (from 2.5 to 4.0 wt%):
1) When C > 3.24 wt%, primary M7C3 forms. Then with the increase of C, the Cr content decreases by 6 wt% (from 57 to 51 wt%) and the Fe content increases by 6 wt% (from 41 to 47 wt%), while other elements’ contents are almost at constant (less than 2 wt%). As known, the hardness of (Cr, Fe)7C3 (Vickers hardness: 2305–2410)13) is higher than that of (Fe, Cr)7C3 (Vickers hardness: 1600–1800).13) This decrease of Cr might lead to a decrease of the hardness of primary M7C3.
2) In eutectic M7C3, the Cr content slightly decreases when C < 3.24 wt% (from 53 to 52 wt%), afterwards it decreases largely by 9 wt% (from 52 to 43 wt%). The Fe content increases linearly by 14 wt% (at C < 3.24 wt%, from 38 to 45 wt%; at C > 3.24 wt%, from 45 to 52 wt%). Other elements’ contents are almost at constant (less than 4 wt%) except Mo which changes from 6.5 wt% for hypoeutectic HCCI to 2 wt% for hypereutectic one. This increase of Fe lead the constitution of M7C3 change from (Cr, Fe)7C3 at C < 3.6 wt% to (Fe, Cr)7C3 at C > 3.6 wt%. It might lead to a decrease in the hardness of eutectic M7C3.
4.2.3. Effect of TiPrecipitation sequence: It was found in pseudo binary phase diagram (Fig. 4(b)), Fig. 8(b) and Table 1 that the precipitation sequence of solid phases doesn’t change with Ti in the range of Ti < 5.4 wt%. It follows,
Alloy L1 (Ti = 0 wt%) to L4 (Ti = 3.36 wt%), alloy Z1 (Ti = 0 wt%) to Z4 (Ti = 1.47 wt%), alloy C1 (Ti = 0 wt%) to C3 (Ti = 2.00 wt%):
MC (With Ti addition)→ primary M7C3 → eutectic M7C3 + FCC
While for alloy B1 (Ti = 0 wt%) to B5 (Ti = 1.68 wt%):
MC (With Ti addition)→ primary FCC → eutectic M7C3 + FCC
When Ti > 5.4 wt%, the primary phase changes from M7C3 to FCC. Thus for alloy C4(Ti = 6.00 wt%):
MC→ primary FCC → eutectic M7C3 + FCC
For alloy L1 to L4, Z1 to Z4 and C1 to C3, with the increase of Ti, the precipitation temperature of MC greatly rises, and the temperature interval between the precipitation of primary M7C3 and eutectic M7C3 + FCC is smaller.
Precipitation amount: From Fig. 5, MC(TiC), primary M7C3 (mainly (Cr, Fe)7C3) and eutectic M7C3 (mainly (Fe, Cr)7C3) are the main carbides. As illustrated in Figs. 10(b1)–10(b4), with the increase of Ti, the amount of MC(TiC) increases largely. At the same time, for hypereutectic alloy (Figs. 10(b1)–10(b3)), the amount of primary M7C3((Cr, Fe)7C3) decreases greatly, although the amount of eutectic M7C3((Fe, Cr)7C3) keeps almost constant. Consequently, the total amount of M7C3 shows decrease while the total amount of carbides shows decrease (Figs. 10(b1)–10(b2)) or almost constant (Fig. 10(b3)) with the increase of Ti from 0 to 3.36 wt%, which is mainly due to the contributions of the decrease of primary M7C3((Cr, Fe)7C3) and the increase of MC(TiC). For hypereutectic alloy (Fig. 10(b4)), the total amount of carbides shows almost constant due to the decrease of total / eutectic M7C3((Cr, Fe)7C3) and the increase of MC(TiC). The available measured data,1,2,3,4) for MC, primary, eutectic and total M7C3 as well as total carbide (M7C3 + MC), fit well with our prediction tendency.
Composition: Considering the hardness control by carbides, the hardness of MC (TiC, Vickers hardness: 3200) is highest, and the hardness of primary M7C3((Cr, Fe)7C3, Vickers hardness: 2305–2410) is higher than that of eutectic M7C3((Fe, Cr)7C3, Vickers hardness: 1600–1800).13) Figure 5 shows the predicted compositions for primary M7C3((Cr, Fe)7C3) and eutectic M7C3((Fe, Cr)7C3) among the main carbides for alloy L1 to L5. It is found that with the increase of Ti (from 0 to 3.4 wt%):
1) In primary M7C3, the Cr content increases by 14 wt% (from 42 to 56 wt%) and the Fe content decreases by 14 wt% (from 56 to 42 wt%), while other elements’ contents are almost constant (less than 3 wt%).
2) In eutectic M7C3, the Cr content increases by 16 wt% (from 34 to 50 wt%) and the Fe content decreases by 16 wt% (from 62 to 46 wt%), while other elements’ contents are almost at constant (less than 4 wt%).
3) It indicates that the increase of Ti changes the M7C3 carbide constitution from (Fe, Cr)7C3 to (Cr, Fe)7C3 in both primary and eutectic M7C3 which will increase the hardness of M7C3 carbide.
4.2.4. Effect of CrPrecipitation sequence: It was found in pseudo binary phase diagram (Fig. 4(c)), Fig. 8(c) and Table 1 that the precipitation sequence of solid phases doesn’t change with the increase of Cr. It follows,
Alloy L3 and L5 (Cr = 16.7–17.4 wt%) , Z4 (Cr = 19.97 wt%), C3 (Cr = 25.0 wt%) :
MC → primary M7C3 → eutectic M7C3 + FCC
With the increase of Cr, the precipitation temperature of MC rises slightly, and the temperature interval between the precipitation of primary M7C3 and eutectic M7C3 + FCC is slightly larger.
Precipitation amount: It is deduced from Fig. 5 that MC(TiC), primary M7C3((Cr, Fe)7C3) and eutectic M7C3((Fe, Cr)7C3 when Cr < 20 wt%, otherwise (Cr, Fe)7C3 ) are the main carbides. As shown in Fig. 10(c), with the increase of Cr, the amount of MC(TiC) is constant. However, the amount of primary M7C3((Cr, Fe)7C3) increases at Cr < 20 wt% then decreases at Cr > 20 wt%; and the amount of eutectic M7C3 changes oppositely to the primary one. Consequently, the total amount of carbides shows a slight increase with the increase of Cr from 17.4 to 25.0 wt%, which is mainly the contribution of eutectic M7C3((Fe, Cr)7C3).
Composition: Figure 5 shows the predicted compositions for primary M7C3((Cr, Fe)7C3) and eutectic M7C3((Fe, Cr)7C3 when Cr < 20 wt%, otherwise (Cr, Fe)7C3) among the main carbides for alloy L5, L3, Z4 and C3, considering the hardness control by carbides. It is found that with the increase of Cr (from 17.4 to 25.0 wt%):
1) In primary M7C3, the Cr content increases by 13 wt% (from 49 to 62 wt%) and the Fe content decreases by 11 wt% (from 49 to 38 wt%), while other elements’ contents are almost at constant (less than 3 wt%).
2) In eutectic M7C3, the Cr content increases by 19 wt% (from 41 to 60 wt%) and the Fe content decreases by 14 wt% (from 54 to 40 wt%), while other elements’ contents are almost at constant (less than 4 wt%).
3) It indicates that the increase of Cr changes the M7C3 carbide constitution from (Fe, Cr)7C3 to (Cr, Fe)7C3 in both primary and eutectic M7C3, which will increase the hardness of M7C3 carbide.
4.3. Hardness of High Cr Cast IronsThe matrix of HCCI, FCC phase, is mainly in charge of its toughness; while the carbides in the structure act mainly on its hardness. Based on the predicted carbide precipitation amount (Fig. 10) and carbide composition and constitution (Fig. 5), the hardness of high Cr cast irons is evaluated quantatively using the definition of total hardness index of high Cr cast iron and with the help of Vickers hardness data for carbides (Vickers hardness of (Fe,Cr)7C3 is 1600–1800, Vickers hardness of (Cr, Fe)7C3 is 2305–2410, and Vickers hardness of TiC is 3200).13)
The total hardness index of high Cr cast iron drawn in Fig. 11 is defined as the contributions of the hardness of MC and M7C3 carbides and weighted by their predicted volume fraction. Corresponding to the varied constitution of primary and eutectic M7C3 as shown in Fig. 5, different values of Vickers hardness are adopted ((Vickers hardness of (Fe,Cr)7C3 is 1600–1800, Vickers hardness of (Cr, Fe)7C3 is 2305–2410). The lower limit of hardness index labeled ‘min’ and upper limit ‘max’ denote the range of the hardness index, 400–900, using the minimum and maximum Vickers hardness for each carbide constitution.

Effects of (a) C, (b) Ti and (c) Cr content on the hardness index of high Cr cast irons. Total hardness index is the contributions of MC, Primary M7C3 and eutectic M7C3 carbide’s hardnesses weighted by their volume fractions as seen from Fig. 10. Carbides’ Vickness hardness is shown in Table 2. (Online version in color.)
| Alloy | Carbide | Compositions (wt%) | Precipitation amount in volume fraction (–) | Note | Vickness hardness13) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Cr | Ti | Ni | Mo | Mn | Si | Fe | |||||
| L1 | MC | – | – | – | – | – | – | – | – | – | ||
| PrimaryM7C3 | 8.66 | 38.26 | – | 0.17 | 0.66 | 1.15 | – | 51.10 | 0.26 | (Fe, Cr)7C3 | 1600–1800 | |
| EutecticM7C3 | 8.60 | 31.49 | – | 0.19 | 1.15 | 1.57 | – | 57.00 | 0.12 | (Fe, Cr)7C3 | ||
| M3C | 6.68 | 8.57 | – | 0.52 | 1.91 | 2.73 | – | 79.59 | 0.11 | |||
| L2 | MC | 18.40 | 16.60 | 61.90 | 0.01 | 2.85 | 0.05 | 3.9E-05 | 0.26 | 0.02 | TiC | 3200 |
| PrimaryM7C3 | 8.69 | 43.96 | – | 0.12 | 0.77 | 1.14 | – | 45.31 | 0.12 | (Fe, Cr)7C3 | ||
| EutecticM7C3 | 8.61 | 36.99 | – | 0.15 | 1.85 | 1.78 | – | 50.63 | 0.17 | (Fe, Cr)7C3 | ||
| M3C | 6.59 | 7.40 | – | 0.44 | 4.88 | 4.87 | – | 75.80 | 0.24 | |||
| L3 | MC | 18.30 | 14.10 | 65.10 | 0.01 | 2.32 | 0.05 | 3.8E-05 | 0.21 | 0.03 | TiC | |
| PrimaryM7C3 | 8.71 | 47.39 | – | 0.11 | 0.70 | 1.23 | – | 41.86 | 0.11 | (Cr, Fe)7C3 | 2305–2410 | |
| EutecticM7C3 | 8.61 | 40.26 | – | 0.14 | 2.31 | 2.12 | – | 46.56 | 0.18 | (Fe, Cr)7C3 | ||
| M3C | 6.47 | 0.07 | – | 0.16 | 8.18 | 11.40 | – | 73.70 | 3.8E-5 | |||
| L5 | MC | 18.30 | 14.10 | 64.90 | 0.01 | 2.48 | 0.04 | 2.0E-05 | 0.21 | 0.03 | TiC | |
| PrimaryM7C3 | 8.70 | 46.20 | – | 0.11 | 0.75 | 1.18 | – | 43.10 | 0.10 | (Cr, Fe)7C3 | ||
| EutecticM7C3 | 8.60 | 39.00 | – | 0.13 | 2.38 | 2.09 | – | 47.80 | 0.19 | (Fe, Cr)7C3 | ||
| M3C | – | – | – | – | – | – | – | – | – | |||
| L4 | MC | 18.00 | 10.30 | 69.20 | 0.01 | 2.24 | 0.04 | 4.0E-05 | 0.18 | 0.06 | TiC | |
| PrimaryM7C3 | 8.73 | 50.83 | – | 0.09 | 0.75 | 1.21 | – | 38.38 | 0.05 | (Cr, Fe)7C3 | ||
| EutecticM7C3 | 8.65 | 45.31 | – | 0.11 | 2.13 | 1.97 | – | 41.83 | 0.18 | (Cr, Fe)7C3 | ||
| M3C | – | – | – | – | – | – | – | – | – | |||
It is known from Fig. 10 that M7C3 carbide dominates in the amount of total carbides. With the increase of C and Cr content, the amount of M7C3 and total carbides increases. Although the increase of Ti decreases the total amount of the carbides as a direct result of decreasing the amount of M7C3 but increasing the amount of MC, the total hardness of the alloy is compensated since the Vickers hardness of MC is much higher than that of M7C3. As a result, the maximum and minimum hardness index of high Cr cast iron increase with the increase of C, Ti and Cr contents except the case at Ti = 0. In that case, there is no hardest MC, but the primary and eutectic M7C3 ((Fe, Cr)7C3) occupies much higher amount of carbides than other Ti addition cases. Within the composition concerned, the highest hardness index, 850–900, is obtained at the 4 wt%C, 1.5 wt%Ti and 25 wt% Cr content. Considering the moderate addition of the alloy element, the addition of Cr with 17–20 wt% content might be recommended.
In Fe–C–Cr–Ti–Mn–Mo–Ni–Si Ti-added HCCIs, the nature of carbides, such as the kinds, the precipitation sequence, the amount and the compositions, during the solidification and its effects on the hardness of the alloy are discussed as a function of C, Ti and Cr content by the help of Partial Equilibrium (PE) approximation, DSC thermal analyses and EDX analyses. It indicates that,
(1) Carbide precipitation kind, sequence and amount. The main carbides formed during the solidification are MC, primary M7C3 and eutectic M7C3 within the variations of C in 2–5 wt%, Ti in 0–4 wt% and Cr in 15–25 wt% for Fe–C–Cr–Ti -(0.4~2.0)wt%Mn -(0.7~3.0)wt%Mo -(1.~2.6)wt%Ni -(0.6~1.5)wt%Si alloy. The primary and eutectic M7C3 are distinguished as two kinds of carbides in the present paper due to their distinguished compositions while other researchers didn’t distinguish them.
The precipitation sequence of the eutectic structure in HCCIs starts from the precipitation of FCC prior to the eutectic M7C3, they don’t precipitates simultaneously as usually expected.
The increase of C doesn’t change the amount of MC while greatly increases the amount of total M7C3 carbide through precipitating not only eutectic M7C3 but also primary M7C3 carbide. The increase of Cr shows little effects on the amount of MC and M7C3 carbides. Contrarily, the addition of Ti greatly decreases both the amount of primary M7C3 carbide when C > 3.24 wt% and the amount of eutectic M7C3 when C < 3.24 wt% by the replacement of MC carbide.
(2) Carbide composition. Both the compositions of primary M7C3 and eutectic M7C3 carbide change greatly with C, Ti and Cr content. The Cr composition in primary and eutectic M7C3 carbide increases with both Cr and Ti contents but decreases with C content, while the Fe composition in M7C3 carbide shows the contrary tendency. The increase of Cr content increases the hardness of M7C3 carbide.
(3) Carbide hardness. The hardness index of the HCCIs, which is comprehensively evaluated by the summation of the contributions of the Vickness hardness of MC, primary and eutectic M7C3 carbides with predicted precipitation amount and constitution, alters from 400 to 900 with the C, Ti and Cr content concerned. The maximum and minimum hardness index of high Cr cast iron increase with the increase of C, Ti and Cr contents. The highest hardness index, 850–900, is reached at 4 wt% C, 1.5 wt% Ti and 25 wt% Cr content.
Partial Equilibrium approximation, based on the thermodynamic equilibrium calculation and a reliable database, gives more reasonable prediction on the solidification behavior in multi-component alloys, even for the case of large amount of precipitated carbides.
The work is supported by 111 Project [No.B07015] and Fundamental Research Funds for the Central Universities [N100409004], China.