2014 Volume 54 Issue 4 Pages 994-996
2014 Volume 54 Issue 4 Pages 994-996
The demand for iron ore in Malaysia has increased steadily over the years. Malaysia has abundant low grade iron ore which has not been utilized as raw materials in iron making. There is a commercial potential to utilize local iron ore in blast furnace process of iron making, since a mini blast furnace with capacity of 0.5 million tons/year was constructed in 2012.1) Problems related to utilization of low grade iron ore in iron making process are the presence of combined water, impurities and unfavourable mechanical properties.2,3,4) Moreover, the utilization of low grade iron ore is not efficient since it requires extra energy consumption of coke as energy source. On the other hand, as the reduction process in iron making is driven with the carbothermic boudouard reaction and hydrogen reaction, there is a possibility to replace coke and natural gas with agro-industry residues which could provide both, carbon and energy for the reduction process.5,6,7)
Beneficiation of low grade iron ore without further treatment is not appropriate since the iron oxide in the ore has weak magnetic properties.8,9) Thus, treatment of ore to improve the magnetic properties prior to upgrading process through magnetization technique is necessary. Many research works related to the prospective study of magnetic properties of iron ore have been reported.8,9,10) Wu et al. (2012) investigated the effect of pine sawdust as energy source onto magnetic properties of goethite ore through reduction roasting. Meanwhile, Pramusanto et al. (2011) also used reduction roasting as the method to study the effect of coal/ laterite ore ratios on the magnetization of the pellet. On the hand, Hadi et al. (2003) studied the magnetic properties of laterite ore before and after reduction process using CO/CO2 mixed gas as the source of energy. These studies show that reduction process could improve the magnetic properties of weak magnetic iron oxide into high magnetic iron oxide. However, the effect of magnetic properties of low grade iron ore through reduction roasting using oil palm empty fruit bunch as energy source has not been reported. The purpose of this work is to investigate the effect of reduction roasting using oil palm empty fruit bunch as energy source on the magnetic properties of Malaysian iron ore.
Malaysian iron ore taken from Chini, Pahang state and oil palm empty fruit bunch (EFB) taken from Bentong, Pahang state were employed as raw materials in this work. Iron ore and EFB were ground using ball mill and sieved into small particles with size of less than 150 μm and 500 μm, respectively. The ore used contains 58.1 mass% of Fetotal and 3.75 mass% of combined water.1) Meanwhile, the EFB mainly consists of carbon, hydrogen, oxygen and nitrogen as shown in Table 1. The weight loss of EFB heated at 200–350°C is 57.78 mass%, due to the decomposition of organic matter into synthetic gases.11,12,13) The ground iron ore and EFB were mixed using mixer with additional minute amount of water. Then, the mixed powder was fabricated into spherical pellet with diameter of 10 mm by using conventional hand rolling. The composition of pellet varies with EFB content from 10 to 40 mass%. The pellet was dried at 110°C for two hours in an electric oven to release moisture. Reduction roasting experiment was carried out using an electric tube furnace at 600–900°C for 30 minutes at 10°C/min heating rate. Argon gas was constantly flowed throughout the experiment until the cooling process at atmospheric condition. A mass flow controller was used to control the flow rate of the gas at 100 ml/min. For each experiment, the weight loss of each pellet was measured before and after heating. The fractional reduction was estimated based on the different between initial and final weight of pellet as detailed elsewhere.1,14)
| Biomass | Weight loss | Proximate analysis | Ultimate analysis | ||||
|---|---|---|---|---|---|---|---|
| Volatile matter | Fixed carbon | Ash | C | H | O | ||
| EFB | 57.78* | 69.42 | 16.15 | 4.25 | 45.64 | 6.19 | 48.17 |
The iron ore before and after reduction tests were crushed prior to magnetization test using vibrating sample magnetometer (VSM) to characterize the magnetic properties of the sample by applying elevated magnetic field on the sample. The magnetization is defined as magnetic moment per gram sample and it depends on the magnetic field (H) applied to the sample. The condition when the applied magnetic field does not affect the magnetization of the sample is called as saturation and the magnetization value at this point is defined as magnetic saturation (Ms). About 1 gram of powder iron ore was prepared for the measurements of magnetization which were conducted at room temperature (26°C) under a maximal magnetic field up to 20 kOe.
Figure 1 shows the fractional reduction of iron ore at different temperature for various EFB content. The fractional reduction increased with increasing temperature and EFB content. In case of heating at 600°C, only small change in fractional reduction was observed when 10 to 30 mass% of EFB was present in the pellet, instead, there is a great significant increment in fractional reduction when 40 mass% of EFB was present. Moreover, the fractional reduction improved significantly up to 22 mass% when the pellet was heated at 900°C. In most cases, the reduction at 900°C proceeds up to 20% as a result of the presence of the reducing gases formed by the decomposition of volatile matter.15,16) As the temperature increased, the stepwise reduction reaction of iron oxide continues steadily and the diffusion of CO and H2 through the pellet which was initiated from the gasification of EFB produces favourable conditions and increases the concentration of reducing gases. The concentration of synthetic gases increase with increasing temperature and the mechanisms during gasification of EFB at 600–900°C produced 10–27 vol% of H2, 21–33 vol% of CO and 5–14 vol% of CH4.17) The iron content was improved by almost 12% from 58.1 mass% in the original ore to 64.9 mass% after reduction roasting using 40 mass% of EFB.

Fractional reduction of iron ore with temperature at different composition.
Figure 2 shows XRD patterns of iron ore before and after reduction test for pellet containing 40 mass% EFB. It is apparent that iron in the original iron ore mainly present as goethite and hematite with existence of impurities in the form of silicate minerals, quartz (SiO2). The quartz peaks remained in the ore as temperature increases. The goethite and hematite in the original iron ore disappeared after being heated at moderate temperature and got transformed into magnetite and wustite at 600 to 800°C. On the other hand, clear peaks attributed to metallic iron appeared when the ore was heated at 900°C with predominant magnetite and wustite peaks. These results apparently indicate that the dehydration of combined water and the reduction reaction by syngas occurred concomitantly during iron ore reduction roasting conditions. The peaks from goethite and hematite were successfully reduced and emergence of strong peaks of magnetic phase comprising magnetite and metallic iron as well as the appearance of wustite peaks simultaneously.
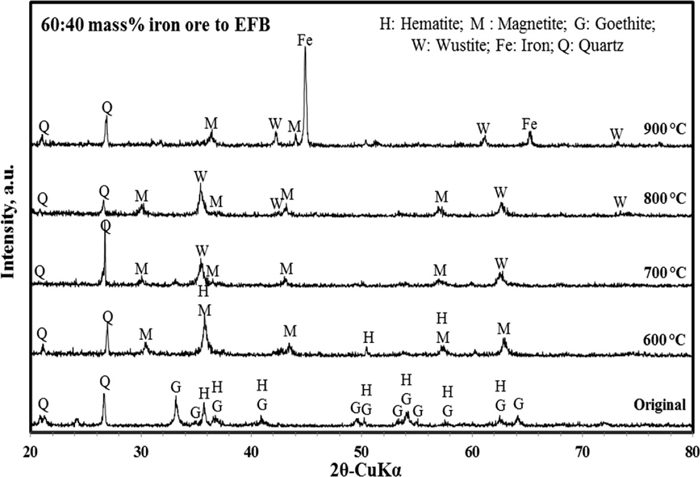
XRD patterns of iron ore before and after reduction roasting.
Figure 3 shows the magnetization curves of iron ore before and after reduction roasting at different temperatures with applied magnetic field for pellets containing 40 mass% EFB. The Ms value for original iron ore was obtained at 1.11 emu/g showing the low magnetic properties since the main components in the ore are goethite and hematite which have low magnetic properties. The slope rises during applied magnetic field up to 2 kOe revealing the magnetic susceptibility of the ore, and the value measured from the slope is 0.03 × 10–3 emu/g. This value is close to the magnetic susceptibility of several ores containing goethite which is reported around 0.01 × 10–3 to 0.02 × 10–3 emu/g.8,9,10,18,19)
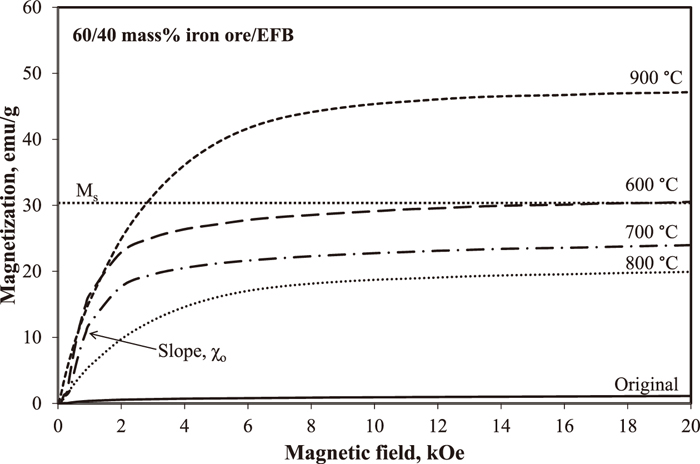
Magnetization of iron ore before and after reduction roasting at different temperature.
Meanwhile, it is obvious that the addition of EFB shows enhancement in the magnetic properties of iron ore compared to original ore and the magnetization was improved with increasing temperature. The curves were drastically improved up to 5 kOe and the gradients become small as the ores reached the saturation condition. At 600°C, the Ms value increased up to 30.7 emu/g at applied magnetic field of 3 kOe with magnetic susceptibility of 28.13 × 10–3 emu/g. However, the magnetic susceptibilities of iron ore heated at 700 and 800°C decreased to 21.99 × 10–3 and 17.94 × 10–3 emu/g, respectively. The magnetic susceptibility of ore heated at 600°C is higher than 700 and 800°C due to the presence of magnetite which has been classified as having strong magnetic phase. However, the magnetic properties of the iron ore heated at 700 and 800°C decrease due to the increase in wustite peaks, and wustite has been categorized as paramagnetic material or known as weak magnetic phase. With increasing temperature up to 800°C, more wustite peaks were produced which reduce the magnetization of iron ore. Thus, it can be concluded that the presence of magnetite would enhance the magnetic properties of iron ore, while wustite dominance would decrease the magnetic properties of iron ore.
On the other hand, magnetic saturation slightly increased up to 47.1 emu/g at 900°C when applied magnetic field was up to 5 kOe and the magnetic susceptibility is 44.6 × 10–3 emu/g. It could be explained that goethite was successfully reduced into magnetite and metallic iron with small amount of wustite, consequently improved the magnetic properties of the ore. Thus, in the presence of 40 mass% of EFB, the magnetization curves for all ores completely become ferromagnetic. The magnetization of iron ore was improved as increasing the number of ferromagnetic phases in the iron ore after heating up to 900°C. The magnetization data of iron ore from the magnetization curves are summarized in Table 2.
| Sample | Magnetic susceptibility χ 10–3 (emu/g) | Saturated magnetization Ms (emu/g) |
|---|---|---|
| Original | 0.03 | 1.11 |
| *Natural Hematite | 0.02–0.03 | – |
| Heated at 600°C | 28.13 | 30.7 |
| Heated at 700°C | 21.99 | 23.9 |
| Heated at 800°C | 17.94 | 19.8 |
| Heated at 900°C | 44.6 | 47.1 |
| *Natural Magnetite | 35.98–45.14 | – |
The magnetic properties of original iron ore was improved by reduction roasting through removal of combined water and formation of strong magnetic minerals such as magnetite and metallic iron. Strong magnetic iron oxides are easy to be attracted and separated by magnetic force during magnetic separation process, while the weak magnetic iron oxides which are considered as paramagnetic materials are difficult to be attracted into magnetic force. Prior to the magnetic separation process for industrial practice, it is obvious that the original ore and iron ore heated at 700 and 800°C are not suitable candidates for upgrading treatment. However, iron ores heated at 600 and 900°C are suitable for further upgrading process using magnetic separator, since they were successfully magnetized into strong magnetic minerals through reduction roasting process.
The reduction roasting test of Malaysian iron ore using oil palm empty fruit bunch has been conducted. The results show the reduction degree tends to increase as temperature increases up to 900°C and EFB content up to 40 mass%. The low magnetic phases; goethite and hematite in the original ore were converted and improved into strong magnetite at 600°C with magnetic saturation of 30.7 emu/g and 28.13 × 10–3 emu/g of magnetic susceptibility. The magnetic properties of iron ores were improved at 700 and 800°C, but the presence of wustite lowers the magnetic properties compared to 600°C. Increasing temperature up to 900°C enhances the magnetic properties of iron ore due to the presence of magnetite and metallic iron which are classified as strong magnetic materials. The magnetic saturation of iron ore increases up to 47.1 emu/g and its magnetic susceptibility enhances by 40 times of 44.6 × 10–3 emu/g from the original iron ore. Therefore, the reduction roasting of low grade iron ore using oil palm empty fruit bunch could improve the magnetization of low grade iron ore and assist further upgrading process based on optimal magnetic properties of iron ore.
This research was supported by IIUM through a Research Matching Grant Scheme (RMGS 11-004-0017) and MOSTI Technofund (TF1011D220). We acknowledged Aras Kuasa Sdn Bhd for providing iron ore and FELDA Mempaga mill plant, Pahang Malaysia for the supply of oil palm empty fruit bunch.