2014 Volume 54 Issue 6 Pages 1256-1265
2014 Volume 54 Issue 6 Pages 1256-1265
The effect of alumina and silica on the reaction behavior of carbon composite pellet was investigated by thermogravimetric analysis at 1273 K (1000°C) and 1373 K (1100°C). X-ray diffraction was used for the phase analysis of iron oxide changing from hematite to reduced iron. The overall reaction was divided into three stages according to phase transformation of iron oxide. CO and CO2 from off-gas data was used for kinetic analysis and for understanding reaction mechanism. The Boudouard reaction was largely influenced by alumina and silica that changed CO gas concentration resulting in different reduction behavior of the pellets. Alumina increased the reaction rate of carbon composite pellet while silica decreased the overall reaction rate.
Currently, various beneficiation methods are being developed to process clay-rich or lower grade iron ore in an attempt to meet the rapidly growing demand of steel industry. Among them, wet processing,1) reverse flotation2) and SFCA sinter phase research3) are feasible examples. Impurities, such as quartz, alumina-containing minerals and phosphorus found in iron ore are usually regarded as detrimental by many iron and steelmaking operators. However, despite all the efforts, the beneficiation process will become more difficult and the cost will rise in the near future. Especially, high alumina content in raw material is an inevitable situation for blast furnace operation as well as for direct reduction coupled with electric arc furnace production. There have been numbers of studies on high alumina blast furnace process in terms of slag volume,4) slag viscosity5,6,7) and sulfide capacities.8) Characteristics of conventional blast furnace type slag i.e. CaO–SiO2 (–MgO)–Al2O3 system are known to be largely influenced by basicity (C/S), Al2O3 content and additional MgO amount.9) It was confirmed that the slag structure as well as primary phase field were major influence on the physical properties of molten slag systems.9) Meanwhile, comprehensive studies on carbon composite agglomerate (CCA) used for ITmk3®-Midrex process have been carried out from the view point of carburization, metallization and effect of slags.10,11) In CCA system, iron oxide reduction and iron melting were much improved by Fe–C melt due to the faster mass transport according to Kim et al.,12,13) The role of slag on melting and separation of iron was also clarified from the previous studies.14,15) However, reduction behavior of carbon composite pellet specifically the influence of slag composition is not well understood yet. The influence of CaO–SiO2–Al2O3 ternary slag system on the reduction of carbon composite pellet was studied in terms of reaction kinetics16) as well as morphological properties17) at temperatures between 1623 K (1350°C) and 1773 K (1500°C). According to the results, melting behavior of different slag system affected the morphology of the pellet which significantly changed the reaction rate during reduction.16,17) Individual effect of alumina and silica on the reduction of carbon composite pellet was investigated at 1473 K (1200°C) in the previous study conducted by the authors.18) Interestingly, alumina increased the reaction rate by increasing surface area while silica decreased the reaction rate by lowering surface area of pellet samples. Although the carbon composite pellet samples in the previous studies16,17,18) represent feed material for rotary hearth furnace (RHF) process, clarifying its reaction mechanism is also very important for blast furnace operation as the process of oxygen removal from iron oxide by solid carbon is basically equal to the direct reduction (solution loss) occurring between iron ore and coke. Therefore, current experimental condition represents not only the reactions between solid carbon and solid iron oxide in upper part (shaft) blast furnace operation, but also the feasibility of low temperature RHF process. In the present study, the influence of alumina and silica on the reduction of carbon composite pellet was investigated by thermogravimetric analyzer (TGA) at 1273 K (1000°C) and 1373 K (1100°C). X-ray diffraction was used for the phase analysis of iron oxide changing from hematite to reduced iron in order to confirm TGA results. CO and CO2 from off-gas analysis assisted with kinetic data to clarify reaction mechanism.
For the sample preparation, hematite powder (≥99%, <5 μm), synthetic graphite flake (≥99.99%, ≤150 μm) and reagent grade CaO, SiO2 and Al2O3 chemicals were dried in air at 473 K (200°C) for 48 hours to eliminate moisture. Chemical compositions of carbon composite pellets with different amount of alumina and silica are shown in Table 1. In the composite pellet, Fe2O3 / CaO ratio was initially fixed at 5.66 to make CF2 (CaO·2Fe2O3) compositions. Lower softening temperature of mixed samples was obtained at this composition. Alumina and silica contents were increased by 5 wt% for the investigation of their impact on reduction behavior. Carbon (graphite) content was fixed as 15% of total weight of pellet that was confirmed as adequate from the previous studies.16,17,18) To obtain homogeneity, powder mixtures of hematite, graphite and oxide system were agitated in a rotating drum for 24 hours. Spherical pellets with 8 mm in diameter were made by hand rolling with drops of water to cause the fines to adhere to each other by liquid bridging. Prepared samples were dried at 473 K (200°C) for 24 hours in order to remove the water bond and allowing solid particles to form physical bonds through capillary and surface forces. Although the binding material was not used, dried pellets showed enhanced compressive strength (≥50 N/m2). Pellets maintained their physical integrity due to the oxides (CaO, Al2O3 and SiO2) included in the system. A schematic of experimental apparatus is shown in Fig. 1. The furnace is heated using Super-Kanthal (MoSi2) elements producing a 125 mm hot zone. The assembly of alumina crucible and quartz holder is hanging at the middle of the alumina tube with suspension wire. Alumina crucible was selected as it did not affect the reaction due to small contact area with pellet. No interaction were found between crucible and pellet after experiments as they separated cleanly. When the samples are introduced into the hot zone, weight losses of sample are measured by the balance and instantly recorded by logged computer. The reduction reaction of carbon composite pellet was investigated by this customized thermogravimetric analyzer (TGA) at 1273 K (1000°C) and 1373 K (1100°C) respectively. High purity nitrogen gas was supplied through the bottom of the furnace at a rate of 1 L/min making inert condition. For complete removal of residual gases, gas was purged for more than 20 minutes. Any secondary reactions were prevented through the elimination of stagnant gases of CO and CO2 in the pellets. The furnace was quickly lifted in 5 seconds positioning the samples at hot zone. Weight loss during a reaction was monitored and logged every 5 seconds by computer via a digital balance. Every sample was held in the furnace over 2 hour to ensure completion of reactions. Off gases evolved from the reduction of oxides were monitored and measured by an infrared gas analyzer (ABB, Advance Optima Series AO2020). The gas concentrations of CO and CO2 were used to explain the reaction mechanism in terms of the effect of Boudouard reaction. PANalytical Xpert Multipurpose X-ray Diffraction System (MPD) was used for X-ray diffraction analysis of reduced pellet samples. Ground powder (≤100 μm) samples were packed into the holder of 25 mm diameter and 5 mm depth. Voltage and current of the machine were set at 45 kv and 40 mA. The XRD patterns were obtained by recording the scattering intensities with Nickel filtered Cu Kα-radiation. The scanning were recorded over an angular range from 10° to 100° using a step size of 0.026° and collected the scattering intensity for 1s at each step.
| Sample | Composition of oxides | Total Weight (g) | ||||
|---|---|---|---|---|---|---|
| CaO | Fe2O3 | Al2O3 | SiO2 | 1273 K (1000°C) | 1373 K (1100°C) | |
| CF2 | 15 | 85 | – | – | 1.3659 | 1.4884 |
| CF2-5A | 14.25 | 80.75 | 5 | – | 1.0548 | 1.0159 |
| CF2-10A | 13.5 | 76.5 | 10 | – | 1.1444 | 0.9346 |
| CF2-15A | 12.75 | 72.25 | 15 | – | 1.1000 | 0.8796 |
| CF2-5S | 14.25 | 80.75 | – | 5 | 1.0096 | 0.9704 |
| CF2-10S | 13.5 | 76.5 | – | 10 | 0.8765 | 1.0520 |
| CF2-15S | 12.75 | 72.25 | – | 15 | 0.8531 | 0.8207 |
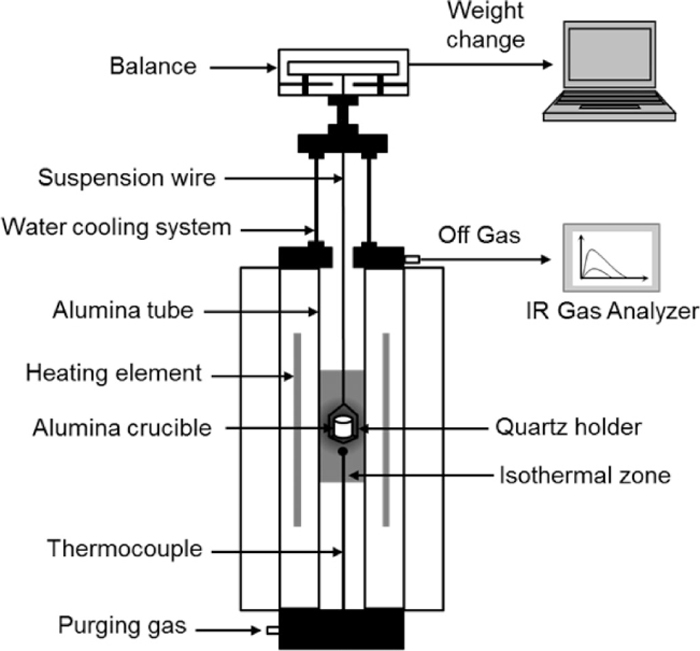
A schematic of experimental apparatus of customised Thermo-Gravimetric Analyzer (TGA).
The reduction of carbon composite pellet takes place through the reaction between hematite and graphite in solid state at 1273 K (1000°C) and 1373 K (1100°C). The experiments were carried using a thermogravimetric analyzer (TGA) by monitoring the weight change of pellet samples during reduction reaction at high temperatures. Moreover, progress of the reaction was investigated through continuous gas analysis with infrared gas analyzer. The reaction mechanism of so-called direct reduction between iron oxide and solid carbon has been studied by numerous investigators.19,20,21,22)
| (1) |
| (2) |
| (3) |
It is well established idea that the Boudouard reaction is significantly slower than the reduction of iron oxide below 1473 K (1200°C),23,24) so the overall reaction rate is controlled by Eq. (3). Boudouard reaction was confirmed to be influenced by different type of carbonaceous materials (coal char, coke and graphite) due to their various surface area as well as hydrocarbon contents.24) According to the study, temperature and catalyst additions (Ca2+) were also major factors affecting activation energy for Boudouard reaction.24) In the current experimental setup, reaction mechanism of hematite reduction by solid carbon can be established as a function of time from in-situ measurement of weight changes of pellet and continuous gas analysis data. The chemical reactions occurring during the reduction of carbon composite pellets are
i) Solid – solid interaction
| (4) |
ii) Gaseous reduction of hematite
| (5) |
iii) CO regeneration
| (6) |
iv) Gaseous reduction of magnetite
| (7) |
v) Gaseous reduction of wűstite
| (8) |
The reactions are occurring in the following order. First, solid carbon particles in composite pellet react with solid hematite as shown in Eq. (4). Although the solid – solid interaction contribute only a small part to the total reduction process, overall reaction cycle is initiated by this reaction. When CO is produced from the Eq. (4), the gas immediately reacts with solid hematite as shown in Eq. (5). This solid – gas reaction generates CO2 gas that is used for a source of carbon solution loss reaction in Eq. (6). The carbon solution loss equation which is typically called as Boudouard reaction plays an important role on the reduction process acting as a driving force of chemical reaction. The Boudouard reaction occurs simultaneously with iron oxide reduction in overall reaction stages. CO gas generated from Eq. (6) subsequently reduces magnetite and wűstite as indicated in Eqs. (7) and (8). Interestingly, magnetite (Fe3O4) exist as an intermediate form of iron oxide between hematite (Fe2O3) and wüstite (FeO) when the pellet was reduced both at 1273 K (1000°C) and 1373 K (1100°C), while it was not observed at 1473 K (1200°C) due to the relatively fast reaction rate.18) The aforementioned reaction mechanism of carbon composite pellet is explained in detail by the combination of gas analysis and reacted fraction data in sections 3.3 and 3.4.
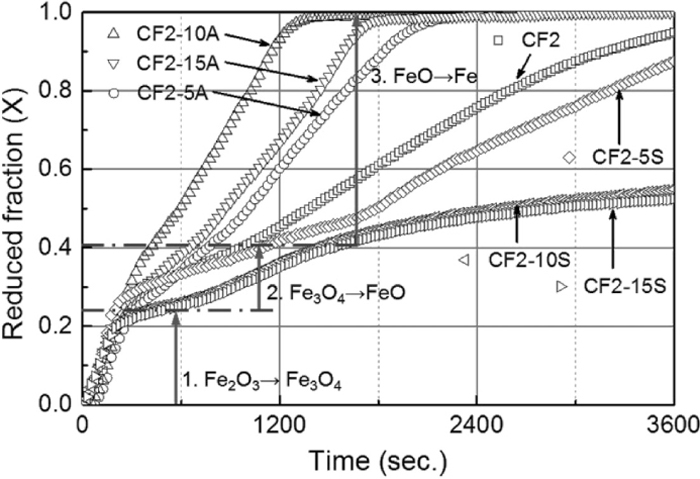
3.2. Temperature Dependence and Activation EnergyIn Fig. 2, the reduced fraction (X) of carbon composite pellets (CF2 samples) are shown as a function of time calculated from the following equation
| (9) |
| (10) |

K is a rate constant derived from the plot of –ln(1 – X) as a function of time at FeO → Fe reaction stages. From Fig. 4, FeO → Fe stage shows the slowest reaction which means a rate controlling step. When plotting with –ln(1 – X) at this stage, the graph showed good linearity with time. The values were calculated at 1273 K (1000°C), 1373 K (1100°C) and 1473 K (1200°C) respectively. A plot of ln(K) against 1/T, shown in Fig. 3, indicates a good linear relationship and yields activation energy of 243 kJ/mol for CF2 sample. According to Moon and Sahajwalla,24) the activation energies for different carbonaceous materials containing briquettes with lime additions were between 106 kJ/mol and 221 kJ/mol. Graphite displays the highest activation energy due to the lowest surface area, while the char with the highest surface area shows the lowest activation energy.24) The presence of the Boudouard reaction catalyst (Ca2+) in the briquettes had lowered the activation energy for all carbonaceous materials. Therefore, the activation energy (243 kJ/mol) obtained from present study is consistent with the previous study carried out at temperatures between 1273 K (1000°C) and 1473 K (1200°C).24)

Arrhenius plot and activation energies for CF2 sample with the comparison of previous research data.24)

Reduced fraction of carbon composite pellets as a function of time at 1373 K (1100°C).
The reduced fraction of carbon composite pellets during reaction at 1373 K (1100°C) is shown in Fig. 4. The overall reaction can be divided into three stages depending on the slope changes of the graphs. First, there is a Stage 1 where Fe2O3 → Fe3O4 reduction occurs. Hematite particles are reacting with solid carbon or CO gas at this stage as shown in Eqs. (4) and (5), then they are rapidly transforming into magnetite. The graph of CF2 sample shows the steepest slope at this stage indicating the fastest reaction rate of hematite / magnetite reduction. Secondly, Fe3O4 → FeO reduction stage is observed between 150 and 300 seconds of reaction time that is equal to Eq. (7). Magnetite exists as an intermediate phase at Stage 2 that contributes to the slightly decreased reduction rate. The result is consistent with a well-known understanding that magnetite is harder to be reduced than hematite due to the higher standard Gibbs free energy as shown in the following equations.25)
| (11) |
| (12) |
Finally, there is a Stage 3 where the reduction of FeO into Fe takes place as seen in Eq. (8). For CF2 sample, wűstite / iron reduction has the slowest reaction rate at this stage as wűstite requires the highest energy for reduction as below.25)
| (13) |
A part from the overall slope changes during reaction process, reduction behavior of pellets at each stage are significantly influenced by added amount of alumina and silica. Samples including alumina (CF2-5A, CF2-10A and CF2-15A) have much faster reaction rate during Stages 2 and 3. The time required for complete reaction varies depending on the alumina content in carbon composite pellets. While CF2 sample takes more than 900 seconds to attain its maximum reduced fraction, samples with alumina require less time. CF2-5A with 5 wt% of alumina takes 600 seconds for complete reaction while CF2-10A with 10 wt% of alumina needs 525 seconds to reach its final state of reaction. Moreover, sample CF2-15A with the highest amount of alumina takes only 450 seconds for complete reduction that is exactly a half of CF2 sample’s case. The faster reaction rate coming from alumina addition can be understood by cation substitution effect between FeOn and Al2O3. In FeOn–Al2O3 binary system,26) FeO·Al2O3 phase has a high melting point at 2093 K (1820°C), while the eutectic between FeOn and FeO·Al2O3 located at 1583 K (1310°C) and no mutual solubility occurs in the sub-system of Al2O3–FeO·Al2O3. According to Roiter,27) Al3+ ion can substitute Fe3+ ion in magnetite from Fe3O4+x through FeAl2O4+x, and spinel phase derived from magnetite with Al3+ substitution can exist with considerable oxygen excess. Therefore, alumina contents in CF2-5A, CF2-10A and CF2-15A samples facilitated magnetite / wűstite reduction by forcing more oxygen to be removed.
In contrast, silica contents from 5 to 15 wt% contributed to the gradual decrease of reaction rate when the carbon composite pellet was reduced at 1373 K (1100°C). In Fig. 4, CF2-5S, CF2-10S and CF2-15S samples are showing slower reaction rate at Stage 2 and 3 when compared to CF2 sample. The slope at Stage 2 is slightly decreases and the reaction becomes more sluggish at Stage 3. At this stage, wűstite / iron reduction should take place, but the reaction seems to be substantially inhibited by silica which is known to have high solubility in FeO–SiO2 melt.28) In FeO–SiO2 binary system, eutectic area at around the fayalite (2FeO·SiO2) compound has low melting temperature at 1451 K (1178°C).29) Although all reactions occur in solid state at 1373 K (1100°C), reduced Fe ion would exist as Fe2+ cation as an intermediate state. Since SiO2 is highly electro-negative to Fe2+ cation considering its acidic characteristics,30) it might have formed a structure that has a higher binding energy. As a result, reactions in CF2-5S, CF2-10S and CF2-15S samples show decreased reaction rate during wűstite / iron reduction stage.
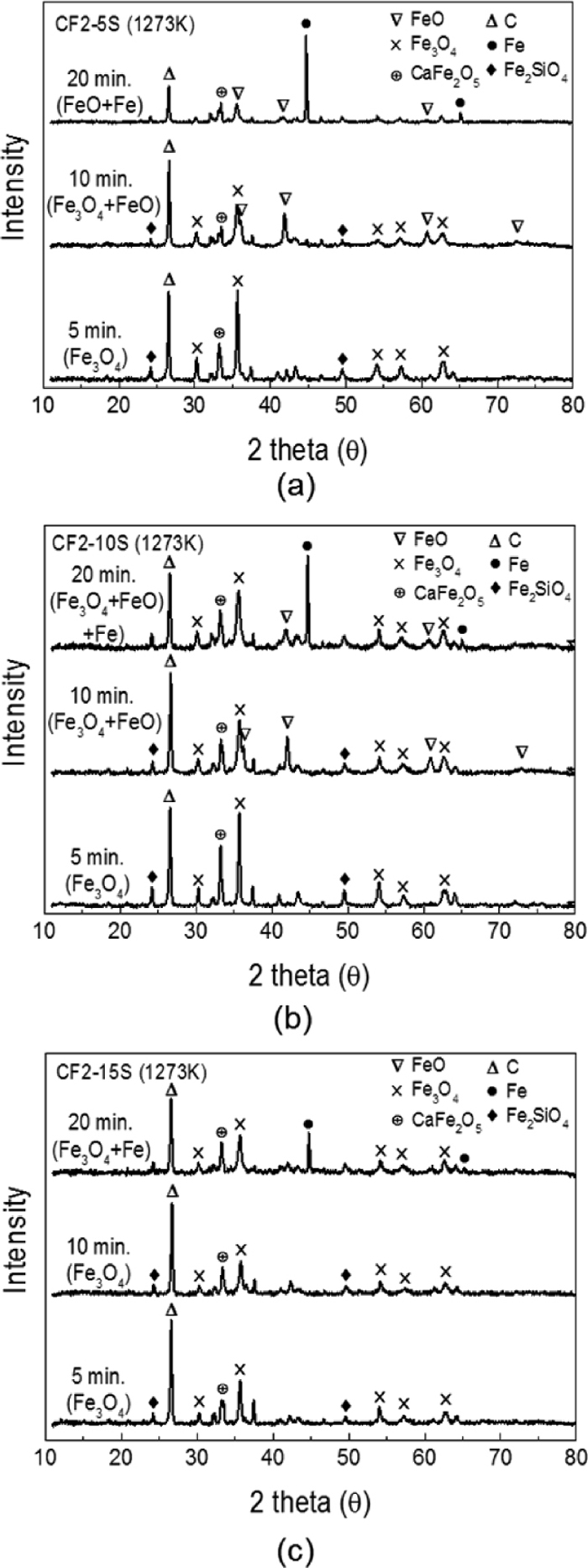
X-ray diffraction analysis is useful for the confirmation of the reduction mechanism of carbon composite pellet that was investigated by thermogravimetric analyzer. During the reduction process, phase transformation of iron oxide is expected to occur from hematite to reduced iron as described earlier. Each sample was collected after 180 second, 300 second and 900 second of reaction at 1373 K (1100°C) and ground into powder (≤100 μm) for XRD measurement. Powder samples were immediately analyzed to prevent oxidation by air. In Fig. 5, the major peaks for carbon, iron, wűstite and magnetite are changing as the reduction proceeds. The results were consistent with the kinetic data from TGA results by showing phase change is in accordance with the reaction stages. CF2 sample is composed of Fe3O4 and solid carbon at 180 second as seen in Fig. 5(a) which is equivalent to reaction Stage 2 in Fig. 4. After 300 seconds, Fe3O4 transforms to FeO with residual solid carbon as the sample proceeds to Stage 3. Reduced iron is observed after 900 seconds when the sample attains the maximum reduction degree. On the other hand, samples with alumina (CF2-5A, CF2-10A and CF2-15A) show faster phase changes. As seen in Figs. 5(b) and 5(c), reduced iron peaks begin to emerge only after 300 second demonstrating much faster reaction rate of alumina samples. Intermediate compound (Ca2(AlFe)O5) were observed as more alumina was added in the system, which supported cation substitution effect. In the case of sample CF2-15A from Fig. 5(d), even though the magnetite is remaining, reduced iron starts to be observed at 300 second indicating a very fast reduction. The residual carbon in composite pellet after reduction can also be used for the estimation of reduction rate. After 900 second of reaction, carbon peaks for CF2-5A, CF2-10A and CF2-15A samples become negligible compared to carbon peak of CF2 sample. This reveals that the carbon solution loss (Boudouard reaction) is easy to occur in alumina containing samples which supports the faster reaction.
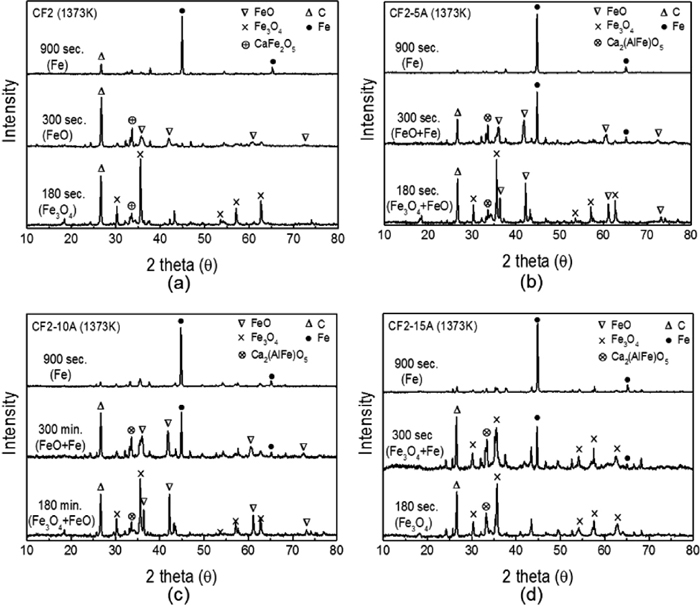
Variation of XRD pattern of reduced pellets with time at 1373 K (1100°C); (a) CF2, (b) CF2-5A (c) CF2-10A and (d) CF2-15A.
In contrast, samples including silica (CF2-5S, CF2-10S and CF2-15S) were confirmed to have slower phase changes than CF2 sample. In Fig. 6, XRD results are shown as a function of reduction time. Similar phase variation is observed in Figs. 6(a) and 6(b) as CF2-5S and CF2-10S sample have the same peak positions at 180 second, 300 second and 900 second of reaction. Magnetite is still remaining until 300 seconds with partially existing wűstite demonstrating slower reaction rates. Moreover, in CF2-15S sample, magnetite remains as primary phase even after 300 seconds. While CF2 sample and alumina containing samples (CF2-5A, CF2-10A and CF2-15A) already initiated wűstite phase transformation in 300 seconds, samples containing silica took more time for magnetite / wűstite reduction. This is due to the formation of fayalite (Fe2SiO4) phase observed from XRD results in Fig. 6. Wüstite easily forms fayalite compound with silica which requires higher energy for reduction.18) Residual carbon at 900 seconds in Figs. 6(a), 6(b) and 6(c) also indicates that reactions at Stage 3 are slower than other samples due to the limited Boudouard reaction in the presence of silica as explained in earlier papers.16,17,18)
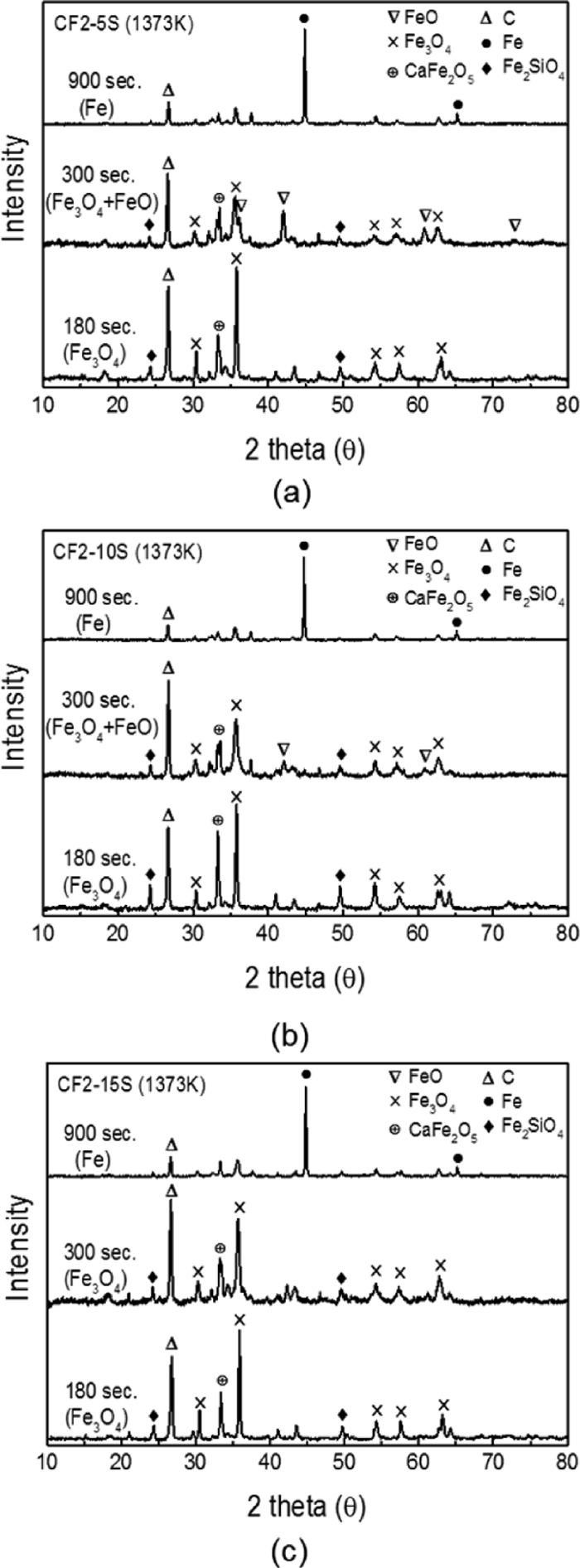
Variation of XRD pattern of reduced pellets with time at 1373 K (1100°C); (a) CF2-5S (c) CF2-10S and (d) CF2-15S.
The increased reaction rate from alumina effect and decreased reaction rate from silica addition could be precisely demonstrated by CO and CO2 analysis from off gas. In Fig. 7, CO and CO2 gas concentrations (vol%) resulting from the reduction of carbon composite pellet at 1373 K (1100°C) are shown as a function of time with reduced fraction. The reaction mechanism elucidated in section 3.1 can be clarified with off-gas analysis. CO2 gas concentration surges in Fig. 7(a) after 30 second when the reaction in Eq. (5) takes place. CO gas begins to increase after 200 second which indicates that CO gas from Eq. (4) was fully consumed by reaction in Eq. (5). When CO is generated from the reaction in Eq. (6), gaseous reduction of Fe3O4 in Eq. (7) also increases producing more CO2 gas. Slightly increased CO2 gas after 200 seconds in Fig. 7(a) could be explained by this mechanism. After 300 seconds, gaseous reduction of wűstite by CO occurs as seen in Eq. (8). Maximum amount of CO gas is produced at this stage providing enough reducing gas for wűstitie / iron reduction. The effect of alumina on CO and CO2 gas generation is revealed in Figs. 7(b), 7(c) and 7(d). In CF2-5A sample, CO gas concentration starts to increase only after 100 seconds and it reaches the maximum at 280 seconds. Generated CO gas from CF-5A is much higher than CF2 sample. From 200 seconds, a gap in reduced fraction between CF2 and CF2-5A becomes wide due to the faster reaction rate of CF2-5A sample from the benefit of increased CO gas amount. This phenomenon is more obvious in the case of CF2-10A and CF2-15A sample. When more alumina is added in carbon composite pellet, the maximum CO gas concentration increases and the CO proportion in total gas increases accordingly. It is shown in Figs. 7(c) and 7(d) that reduction reactions are becoming faster from the point where CO gas concentration begin to rise.
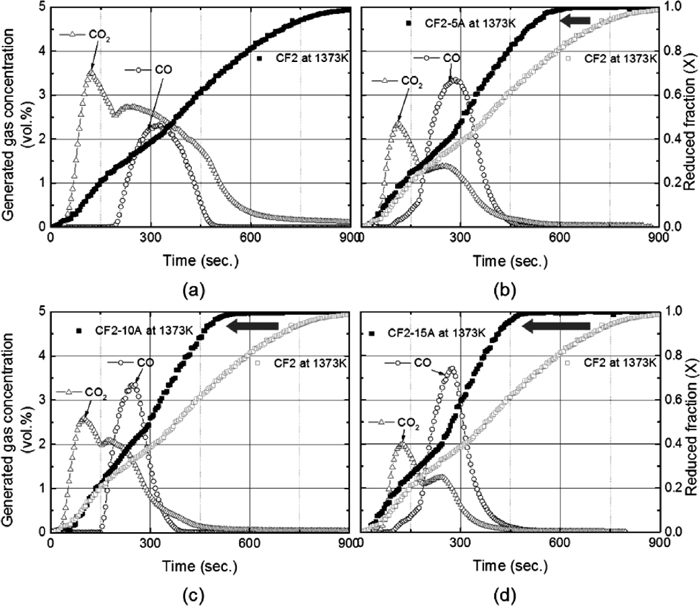
Generated gas concentrations (vol%) in terms of CO and CO2 gases resulted from the reduction of pellets at 1373 K (1100°C) with reduced fraction; (a) CF2, (b) CF2-5A (c) CF2-10A and (d) CF2-15A.
However, analyzed CO and CO2 gas data show opposite results in Boudouard reaction when silica is introduced in the carbon composite pellet system. Figs. 8(a), 8(b) and 8(c) show CO and CO2 gas concentrations (vol%) from off gas measurement and reduced fraction of silica containing pellet samples at 1373 K (1100°C). In CF2-5S and CF2-10S samples, CO gas concentration is observed to be much lower than CF2 sample. Moreover, CO2 gas concentration in these samples gradually decrease, while considerable CO2 amount continues until 600 seconds in CF2 sample as seen from Fig. 7(a). From the reaction mechanism explained in section 3.1, CF2-5S and CF2-10S sample do not seem to produce sufficient amount of CO2 gas from hematite reduction in Eq. (5) that is used for Boudouard reaction in Eq. (6). Thus, reduction reaction of CF2-5S and CF2-10S is limited by the gaseous reduction of hematite and following Boudouard reaction. From Fig. 8(c), Boudouard reaction in CF2-15S sample is more affected by silica by producing lower amounts of CO gas. The reduced amount of CO2 gas from Eq. (5) and decreased CO gas generations from Eq. (6) have resulted decreased reaction rate at 1373 K (1100°C).
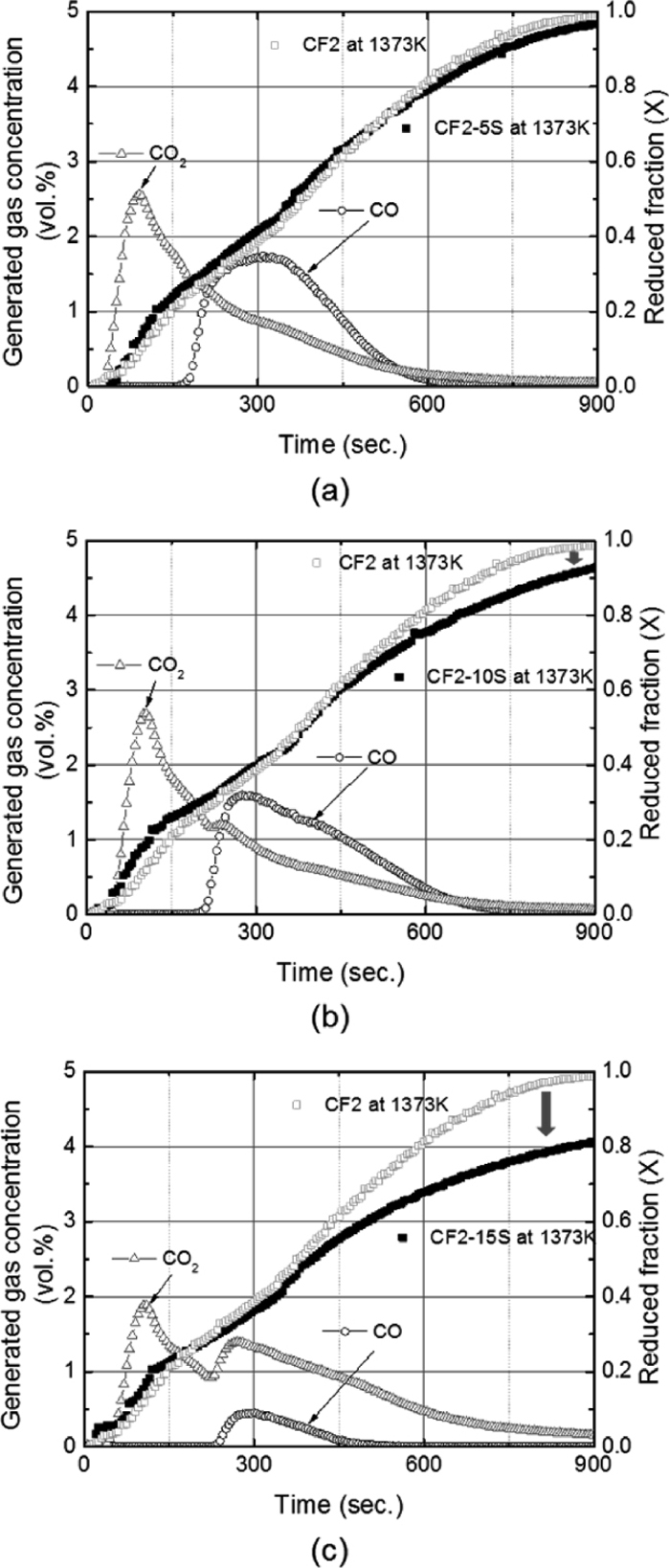
Generated gas concentrations (vol%) in terms of CO and CO2 gases resulted from the reduction of pellets at 1373 K (1100°C) with reduced fraction; (a) CF2-5S (b) CF2-10S and (c) CF2-15S.
In Fig. 9, the reduced fraction of carbon composite pellets during reaction at 1273 K (1000°C) is plotted as a function of time. Like the case of 1373 K (1100°C), the overall reaction is divided into three stages based on the slope changes of the graphs. In Stage 1, hematite / magnetite reduction takes place until 5 minutes that is short period of time considering the overall process. Once hematite transforms to magnetite, reaction rate decreases substantially due to the higher energy needed for reduction as shown in Stage 2. The time period for Stage 2 varies depending on the added amount of alumina and silica. Finally, there is a Stage 3 where wűstite / ion reduction occurs as Eq. (8), which takes the longest reaction time While CF2 sample takes more than 1 hour for complete reduction, samples including alumina (CF2-5A, CF2-10A and CF2-15A) have much faster reaction rate at Stage 2 and 3. CF2-5A with 5 wt% of alumina required 35 minutes for complete reaction, and CF2-10A with 15 wt% of alumina takes only 30 minutes to reach its final state of reaction. Interestingly, effective amount of alumina on reduction reaction is different from the reduction result at 1373 K (1100°C). The higher alumina does not necessarily mean the faster reaction at 1273 K (1000°C). Sample CF2-10A with 10 wt% of alumina takes only 22 minutes for complete reduction that is the minimum among all samples. The fastest reaction rate at 10 wt% of alumina is due to the amphoteric characteristics of alumina.30) A viscosity study on FeOt–Al2O3–SiO2 ternary slag system32) shows that alumina acts as basic as well as acidic oxide depending on the basicity of the melts. In the study, from 5 wt% to 20 wt% of alumina was added and the minimum viscosity was measured at around 10 wt%.32) Likewise, in the present composite pellet system, alumina content up to 10 wt% acts as basic oxide that is considered to be repulsive with Fe2+ ion. However, alumina content above 15 wt% plays an acidic role in carbon composite pellet which demonstrates an attractive force towards Fe2+ ion. Thus, the fastest reaction rate of CF2-10A sample from the amphoteric behavior of alumina can be understood on the basis of this explanation. Whereas, samples with silica show much slower reaction at 1273 K (1000°C). From Fig. 9, the graphs of CF2-5S, CF2-10S and CF2-15S samples have much lowered slopes at Stage 3. This behavior is more evident in CF2-10s and CF2-15S samples since they reach only a half of reduced fraction when compared to other samples. The influence of silica on the reduction of carbon composite pellet is significant at 1273 K (1000°C) as Boudouard reaction is dominant rate controlling step in overall reaction at lower temperatures.
Reduced fraction of carbon composite pellets as a function of time at 1273 K (1000°C).
The influence of alumina and silica on reaction rate of carbon composite pellet at 1273 K (1000°C) was confirmed by X-ray diffraction analysis. Reduced pellet samples were collected and analyzed after 5 minute, 10 minute and 20 minute of reaction at 1273 K (1000°C). In Fig. 10, variation of XRD patterns for CF2, CF2-5A, CF2-10A and CF2-15A are shown with the indication of major peaks. Phase changes of iron oxide in CF2 sample were consistent with reduction stages described in Fig. 9. After 5 minutes, the sample consists of Fe3O4 with residual carbon as shown in Fig. 10(a). As the reduction proceeds, Fe3O4 changes to FeO with carbon at 10 minute. Reduced iron was observed after 20 minutes of complete reduction. Meanwhile, alumina enhances the phase changes of iron oxide in carbon composite pellet. From Figs. 10(b) and 10(c), it is shown that magnetite transforms to wűstite in 5 minutes and reduced iron begins to emerge only after 10 minutes. The result demonstrates the increased reaction rate of CF2-5A and CF2-10A at reduction Stage 1 and Stage 2 at 1273 K (1000°C). This is due to the formation of Ca2(AlFe)O5 compound that is easy to be reduced. This mechanism was explained by cation substitution effect in paragraph 3.3. However, CF2-15A shows slower phase change than CF2-10A sample due to the amphoteric behavior of alumina as explained before. After 10 minutes, mixed peaks of magnetite, wűstite and reduced iron were observed in Fig. 10(d) indicating slower reaction rate of CF2-15A sample compared to CF2-10A sample.

Variation of XRD pattern of reduced pellets with time at 1273 K (1000°C); (a) CF2, (b) CF2-5A (c) CF2-10A and (d) CF2-15A.
In contrast, silica containing samples were confirmed to have slower phase changes than CF2 sample at 1273 K (1000°C). In Fig. 11, XRD results of CF2-5S, CF2-10S and CF2-15S samples are shown as a function of reduction time. After 5 minutes of reduction, magnetite still exists as a primary phase in silica containing samples. Iron oxide phases in CF2-5S sample slowly changes to wűstite in 10 minutes and reduced iron is observed in 20 minutes. The XRD analysis confirms a slightly slower reaction of CF2-5S compared to CF2 as indicated in Fig. 9. Importantly, 10 wt% and 15 wt% of silica included in CF2-10S and CF2-15S have a huge impact on phase changes as seen in Figs. 11(b) and 11(c). Major peak after 10 minutes of reduction is observed to be magnetite and it remains until final stage of a reaction. From the XRD results, reduction stages of magnetite / wűstite and wűstite / iron are confirmed to be significantly influenced by silica effect. Fayalite compound observed in Fig. 11 decreased the overall reaction rate. Gaseous reductions in Eqs. (7) and (8) did not occur sufficiently due to the lack of reducing gas (CO) from the limited Boudouard reaction at this temperature.
Variation of XRD pattern of reduced pellets with time at 1273 K (1000°C); (a) CF2-5S (b) CF2-10S and (c) CF2-15S.
Boudouard reaction is becoming much slower at 1273 K (1000°C) accordingly to previous studies.23,24) When solid carbon reacts with solid iron oxide in carbon composite pellet, the overall reaction is largely influenced by the reaction in Eq. (6). Thus, the influence of alumina and silica on the gasification of carbon needs to be investigated in order to identify the reaction controlling step. The effect of mineral phases on coal combustion for the use of pulverized coal injection (PCI) application was comprehensively studied by Gupta et al.31) According to the study, iron oxide is believed to enhance the coal burning rate, but it has retarding effect when associated with silica.31) Off gas analysis in terms of CO and CO2 assisted with kinetic data to clarify reaction mechanism that was mainly controlled by Boudouard reaction. In Figs. 12 and 13, generated CO and CO2 gas concentrations (vol%) from the reduction of carbon composite pellets at 1273 K (1000°C) are shown with reduced fractions. From Fig. 12(a), small amount of CO gas is generating from reaction of CF2 sample which is significantly lower than 1373 K (1100°C). Limited Boudouard reaction in Eq. (6) provided insufficient CO gas for gaseous reduction in Stage 2 and 3 resulting slower reaction rates in CF2 sample. Alumina containing samples, however, had higher amounts of CO gas as shown in Figs. 12(b), 12(c) and 12(d). Cation substitution effect of Al3+ ion enhances gaseous reduction of magnetite in Eq. (7) which consequently increases Boudouard reaction rate in Eq. (6). CF2-10A sample with 10 wt% of alumina has the highest CO gas concentration among all samples contributing to the fastest reaction rate. The generated gas data is consistent with TGA and XRD results from correlation with Boudouard reaction. In contrast, silica contents from 5 to 15 wt% in carbon composite pellet prevent CO gas generation as seen in Fig. 13. While lower amount of CO gas observed in CF2-5S sample contributed to the reaction rate in Fig. 13(a), negligible amount of CO is produced from CF-10S and CF-15S sample as seen in Figs. 13(b) and 13(c). Boudouard reaction in silica containing carbon composite pellet is limited when iron oxide is included in the system. Since silica has strong attraction with hematite, thus gaseous reduction shown in Eq. (5) is difficult to occur. Limited amount of CO2 generation from Eq. (5) provides insufficient source for Eq. (6). As a consequence, Boudouard reaction acts as a rate controlling step for silica containing samples. Decreased reaction rate of silica containing samples at 1273 K (1000°C) is explained by this reaction mechanism.

Generated gas concentrations (vol%) in terms of CO and CO2 gases resulted from the reduction of pellets at 1273 K (1000°C) with reduction degree; (a) CF2, (b) CF2-5A (c) CF2-10A and (d) CF2-15A.

Generated gas concentrations (vol%) in terms of CO and CO2 gases resulted from the reduction of pellets at 1273 K (1000°C) with reduction degree; (a) CF2-5S (b) CF2-10S and (c) CF2-15S.
The influence of alumina and silica on the reduction behavior of carbon composite pellet at 1273 K (1000°C) and 1373 K (1100°C) was investigated. Following conclusions were made from this study.
(1) The overall reaction of carbon composite pellets was divided into three reduction stages and associated mechanisms were established with respect to time from TGA, XRD and off gas analysis.
(2) Alumina increased the reaction rate by cation substitution effect of Al3+ ion with Fe3+, while silica decreased the reaction rate due to the limited amount of CO gas from Boudouard reaction.
(3) The reaction rate of carbon composite pellets was confirmed to be controlled by Boudouard reaction from gas data. Measured CO and CO2 gas assisted with development of fundamental understanding of reaction mechanisms associated with the influence of alumina and silica.
The authors would like to thank Prof. Yasushi Sasaki at School of Materials Science and Engineering, University of New South Wales and Dr. Sunkwang Jeong at Iron Making & FINEX Research Group, Technical Research Laboratories, POSCO for their insightful advice and encouraging discussion.