2014 Volume 54 Issue 7 Pages 1472-1484
2014 Volume 54 Issue 7 Pages 1472-1484
This paper reviews the global progress on welding consumables for high strength low alloy steel. The numerous aspects, such as the toughness and cleanliness of weld metal, the new removal mechanisms of impurity elements and the crack resistance of weld metal, are discussed. To meet increasing environment requirements, the fumes and life cycle assessment of welding consumables are also discussed. Finally, future trends in the development of welding consumables for high strength low alloy steel are pointed out.
The properties of steel have undergone profound changes in recent years, improving steel cleanliness (i.e. the mass fraction of impurity elements (S+P+O+N+H) in steel) to a lever lower than 250×10–6, and even lower than 100×10–6. Rolling technologies such as the thermo-mechanical control process and deformation induced ferrite transformation can get finer grains, and 2 μm ferrite grains have been obtained in micro-alloyed steel. High strength and toughness steel plates (tensile strength is 600–1000 MPa, and yield strength is 400–800 MPa) are stably produced.1,2) Ultrafine grained steel is widely used. Nanotechnology has been applied in ultrahigh-strength and ultrahigh-toughness steel has been successfully produced. The changes in the technologies of steelmaking and steel rolling are a challenge for welding consumables and the joining technology.
The market for steel and corresponding welding consumables is also influenced by world economy. Table 1 shows outputs in recent years and predictions for the future of steel in the main regions and countries of world.3) According to a research report from Euro Strategy, world steel consumption will amount to more than 2 billion tons in 2017.
| Country | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2017 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| China | 179.7 | 215.1 | 282.9 | 353.2 | 419.1 | 489.3 | 500.3 | 567.8 | 626.7 | 674.0 | 800.0 |
| Japan | 107.8 | 108.5 | 112.7 | 112.5 | 116.2 | 120.2 | 118.7 | 87.5 | 109.6 | 110.0 | 155.0 |
| Russia | 59.8 | 62.7 | 65.6 | 66.1 | 70.8 | 72.4 | 68.5 | 59.9 | 64.0 | 67.0 | 90.5 |
| America | 91.6 | 90.4 | 99.7 | 94.9 | 98.6 | 98.1 | 91.4 | 58.1 | 80.6 | 92.7 | 114.0 |
| India | 28.8 | 31.8 | 32.6 | 45.8 | 49.5 | 53.1 | 55.1 | 56.6 | 66.8 | 93.4 | 160 |
| Korea | 45.4 | 46.3 | 47.5 | 47.8 | 48.5 | 51.5 | 53.6 | 48.6 | 58.5 | 62.6 | 82.7 |
| Asia | 391.7 | 431.7 | 486.0 | 573.5 | 647.5 | 732.8 | 743.1 | 790.0 | 897.9 | 979.0 | 1270.0 |
| Europe | 206.6 | 209.2 | 222.0 | 217.8 | 231.9 | 239.9 | 229.1 | 167.0 | 201.9 | 201.8 | 285.6 |
| North America | 122.9 | 123.2 | 133.0 | 126.0 | 131.5 | 131.2 | 124.5 | 82.3 | 111.8 | 125.2 | 158.1 |
| South America | 40.8 | 43.9 | 46.2 | 45.5 | 45.3 | 48.5 | 47.3 | 37.8 | 43.8 | 45.9 | 62.0 |
| Africa | 15.7 | 16.6 | 16.5 | 17.5 | 18.1 | 19.1 | 17.1 | 15.2 | 17.2 | 17.5 | 24.3 |
| Oceania | 8.3 | 8.0 | 7.7 | 8.7 | 8.7 | 8.6 | 8.4 | 6.0 | 8.2 | 8.5 | 11.6 |
| CIS | 100.4 | 105.9 | 111.2 | 112.8 | 119.6 | 119.5 | 114.3 | 97.5 | 108.4 | 108.0 | 153.3 |
| Middle East | 12.4 | 13.7 | 13.6 | 14.6 | 14.8 | 15.7 | 16.7 | 17.2 | 19.0 | 20.0 | 25.9 |
| World | 898.8 | 952.2 | 1071.5 | 1144.4 | 1247.3 | 1345.8 | 1326.5 | 1219.7 | 1414.0 | 1505.9 | 2000.0 |
In 2009, total consumption and the percentages of different welding consumables in the main regions and countries of world were as shown in Fig. 1. The consumption of welding consumables increased with increased use of steel. The proportion of welding consumables consumed differed because regions may differ in their level of economic and technological development. Overall, the general requirement for world welding consumables tends to be higher strength, higher toughness, more cleanliness, more energy-saving, more environment-friendly, and higher efficiency and automation.4) Welding consumables have developed from traditional Mn–Si alloy system to Ti–B alloy systems that can obtain inclusions with proper feature parameters to induce acicular ferrite (AF) nucleation. New alloy systems in welding consumables can obtain more and finer AF, which could further improve properties of weld metal. High-performance flux cored wire (FCW) and solid wire occupy increasing proportions, while the percentage of stick electrode continues to decrease. Ultra-low hydrogen (<3 ml (100 g)–1) and high toughness welding consumables are widely used in important engineering structures. Welding spatter and fume continue to decrease, while the welding operative performance has continually improved. However, at present the actual contents of impurities and mechanical properties including fatigue strength of weld metal still show a large discrepancy when compared with some steels (see Table 2).
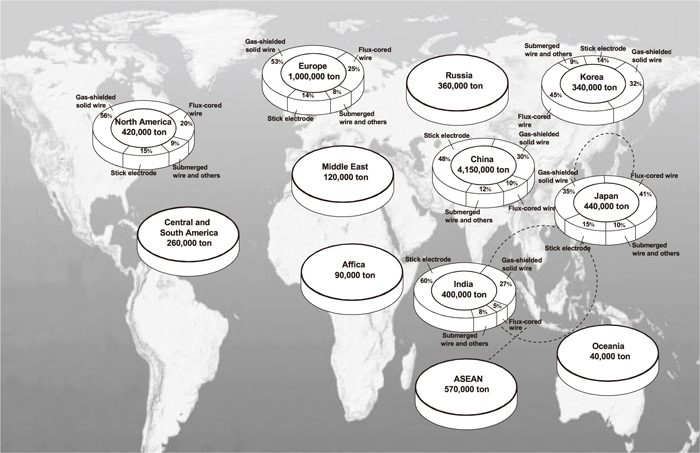
Total consumption and percentage of different welding consumables in main regions and countries in 2009.
| Steel | Typical steel properties | Welding consumable | Typical weld metal properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S/% | P/% | TSa/ MPa | YSb/ MPa | AKVc/J | S/% | P/% | TSa/ MPa | YSb/ MPa | AKVc/J | ||
| Q460 | 0.004 | 0.008 | 720 | 460 | 189@-20°C | E7015 | 0.010 | 0.012 | 490 | 390 | 120@-20°C |
| D36 | 0.009 | 0.011 | 559 | 454 | 184@-20°C | E71T-1 | 0.011 | 0.014 | 540 | 450 | 100@-20°C |
| WDL610D | 0.006 | 0.013 | 740 | 490 | 150@-40°C | E9015-G | 0.008 | 0.013 | 620 | 520 | 110@-40°C |
| N610E | 0.002 | 0.010 | 645 | 600 | 262@-20°C | E71T-1 | 0.008 | 0.011 | 650 | 520 | 65@-20°C |
| X65 | 0.005 | 0.017 | 550 | 470 | 240@-40°C | E71T-1 | 0.006 | 0.150 | 640 | 535 | 53@-40°C |
| X70 | 0.003 | 0.012 | 656 | 532 | 300@-30°C | E71T8-Ni1 | 0.003 | 0.013 | 530 | 415 | 34@-30°C |
| X80 | 0.001 | 0.009 | 825 | 690 | 319@-30°C | E81T8-Ni2 | 0.003 | 0.011 | 585 | 510 | 68@-30°C |
| X100 | 0.001 | 0.010 | 945 | 724 | 255@-60°C | E11018 | 0.008 | 0.017 | 750 | 520 | 98@-20°C |
| X120 | 0.0004 | 0.004 | 1128 | 1087 | 250@-30°C | 0.005 | 0.010 | 941 | 780 | 70@-30°C | |
a TS stands for tensile strength, b YS stands for yield strength, c AKV stands for impact absorbed energy
Phase transformation of high strength low alloy (HSLA) steel weld metal is complicated, and its final microstructure depends on the chemical composition and cooling rate of weld metal. The optimal microstructure for high property weld metal is obtained more AF, duo to the character with fine grain size, high density dislocation in grain, large-angle grain boundaries, and the interlocking structure with each other, which have larger resistance against crack formation and propagation.5) Therefore, the nucleation mechanisms and influencing factors of AF have been investigated. Unfortunately there is no general agreement regarding nucleation mechanisms and control measures of AF until now, so the engineering applications of AF in welding consumables design cannot be controllable. Nevertheless, research on AF nucleation and its influence is still effective in welding consumables design. Also, as the requirements for cracking resistance and fatigue properties of welding joint become higher, diffusible hydrogen (HD) content decreases to 2.8 ml (100 g)–1, and cold and hot crack resistance get further improved. The design of welding consumables performance is now mainly focused on further improving all-position welding operative performance, spatter, fume and high efficiency. Market demands for welding consumables are as shown in Fig. 2.6)
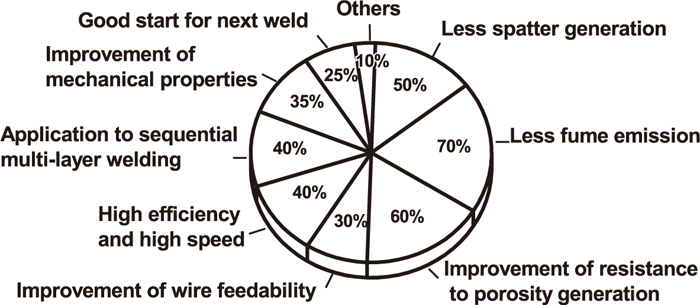
Market demands for welding consumables.6)
There are various opinions about the four mechanisms of AF nucleation in weld metal:5,7,8,9,10,11,12,13)
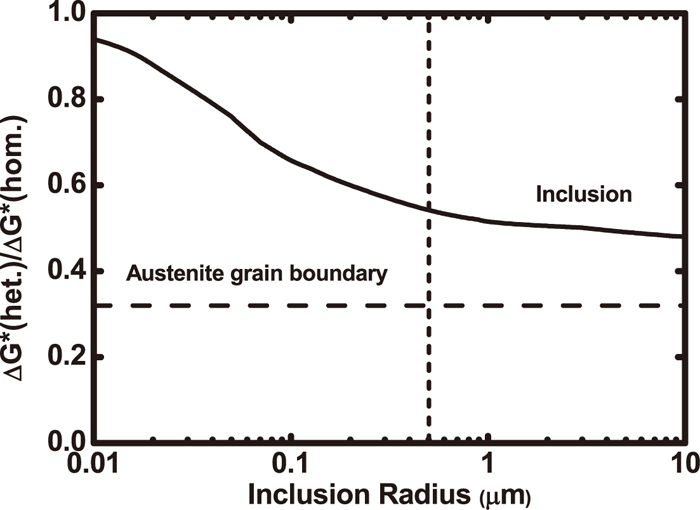
(1) High energy inert matrix nucleation theory: The theory states that inclusions, as the inert nucleation surfaces, reduce the activation energy to promote AF nucleation. However, Sarma et al.11) argued that though providing the external surfaces needed for nucleation, inclusions cannot be equivalent to grain boundaries. The activation energy needed for heterogeneous nucleation of ferrite at the inclusion surfaces is usually higher than that at high angle austenite grain boundaries. However, while activation energy for heterogeneous nucleation of ferrite on inclusions is less than that for heterogeneous nucleation, the ratio will decrease with an increase in the inclusion diameter and reaches less than 1.0 as shown in Fig. 3.7,11,12) Therefore, as centers of heterogeneous nucleation of AF, the size and number of inclusions are important for improvement of weld metal microstructures.
(2) Coherent boundary nucleation theory: The theory states that good lattice matching between AF and inclusions reduces activation energy for nucleation. Due to the constraint of the reproducible orientation relationships between austenite and ferrite, it is difficult for inclusions and ferrite, ferrite and austenite to achieve suitable orientation relationships. Thus, the AF firstly nucleates at an inclusion surfaces. Figure 4 shows the lattice matching for TiC and WC with δ -Fe respectively.11) It is shown that the disregistry between δ -Fe and TiC is lower than that between δ -Fe and WC. Therefore, TiC is more favorable for AF to nucleate.
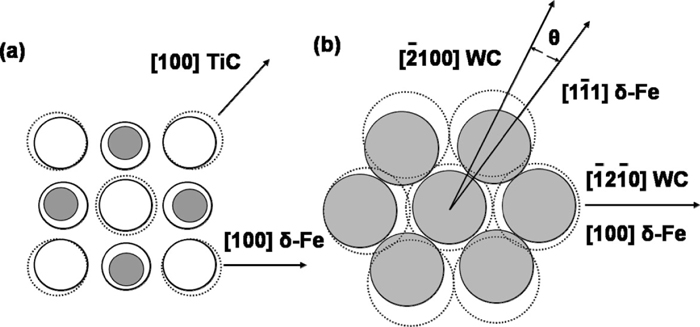
Crystallographic relationship at interface between carbide.11) (a) (100) TiC; (b) (0001) WC and (100) δ -Fe.
(3) Thermodynamics driving nucleation theory: The theory states that inclusions deplete hardening elements such as C, Mn and Si from the austenitic matrix and therefore increase the thermodynamic driving force for ferrite nucleation at inclusion surfaces. The influence of Mn in the Mn-depleted zone (MDZ) to energy barrier for ferrite nucleation at the inclusion surface is shown in Fig. 5.11) It can be seen that activation energy for heterogeneous nucleation of ferrite decreases with a decreased Mn content in the MDZ. Therefore, the nucleation of ferrite with diameters larger than 1 μm nucleates at very low contents (about 0%) of Mn more favorably on the inclusion surfaces than on austenite grain boundaries.
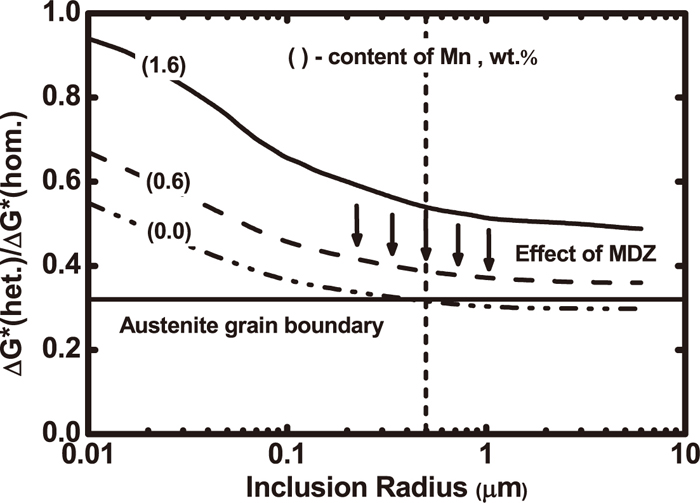
Effects of inclusion size in MDZ on activation energy of heterogeneous nucleation of ferrite with different Mn contents in steels.11)
(4) High strain energy nucleation theory: The theory stated that the thermal expansion coefficients (Δα) of austenite and inclusions are different, so there are thermal stresses on their interface, which can reduce the activation energy for nucleation of ferrite. Figure 6 shows the relationship between Δα of different inclusions in an austenite matrix and the probability of nucleation of AF on inclusions.11) It can be seen that the difference in the Δα between austenite and MnS or Fe-oxides is lower with respect to other inclusions. The tessellated stresses induced by the iron matrix near the inclusions are very small, so MnS and Fe-oxides have no influence on AF nucleation. The probability of AF nucleation on inclusions increases with an increase in the Δα value. Therefore, Mn-Al- or Al-silicates are effective for AF nucleation.
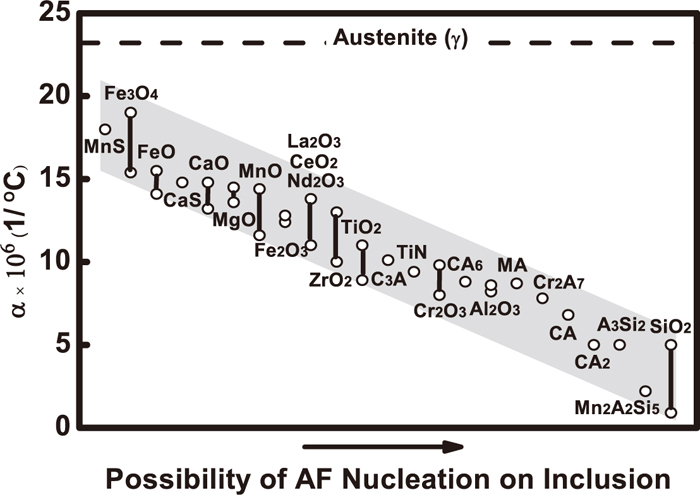
The relationship between thermal expansion coefficient for different inclusions and possibility of AF nucleation.11)
The chemical composition of weld metals is a major factor for controlling fine structures and mechanical properties. According to different research results, the role of different alloying elements on ferrites nucleation can be classified into three kinds: first, to change the γ-α transformation temperature, including increase the austenite zone elements such as C, Mn, Ni, Cu or decrease the austenite zone elements such as Al, Si, V, Cr, Mo, Ti; second, to reduce grain boundary energy for the segregation of solute elements such as B, which can cause an increase of nucleation energy barrier and a decrease of the possibility for ferrite nucleation on an surface of grain boundary surface, and the probability for ferrite nucleation reduces; third, to form a precipitation of inclusion favorable for ferrite nucleation at inclusion surface during the γ-α transformation.
Carbon can increase γ phase zone and delay transformation temperature of austenite, and is also a strong hardening element. The typical range of carbon content in weld metals is 0.05–0.15%. Ramirez14) studied the chemical composition, microstructure and nonmetallic inclusions of high strength steel weld metal, and concluded that when the range of carbon equivalent is 0.26–0.39, deposited metals are mainly ferrites. The fraction of grain boundary ferrite (GBF) decreases with an increase in the fraction of ferrite with second phase (FSP) and AF. The reheated zone of the weld metals transforms to equiaxed polygonal ferrite; with a carbon equivalent of 0.47 or higher, the fraction of lower temperature transformation products including martensite increase in weld metal.
Kim et al.15) studied the effect of Ni on weld metal toughness and results showed that the weld metal impact toughness increased remarkably by an increase of Ni and the value was 118 J at –196°C when Ni content was 16.6%. However, Bhole et al.16) investigated the effect of Ni and Mo additions on weld metal toughness in a submerged arc welding (SAW) of HSLA steel. 2.03–3.75% Ni can decrease the impact toughness of weld metal and increase the fracture appearance transition temperature (FATT); the combination of 2.03–2.91% Ni and 0.7–0.995% Mo may decrease the volume fraction of GBF in weld metal to promote fine AF with high toughness; 0.817–0.881% Mo may accelerate the formation of AF and granular bainite (GB). Weld metal with 0.881% Mo obtains optimum impact toughness at –45°C. The microstructure is mainly comprised of 77% AF and 20% GB.
Avazkonandeh-Gharavol et al.17,18) investigated the effects of 0.14–0.94% Cu and 0.05–0.91% Cr on the microstructure and mechanical properties of Cr–Ni–Cu low alloy steel weld metal. With the increases of Cu and Cr contents in weld metal, AF increases and microstructures become finer in all weld zones while the amount of primary ferrite (PF) and FSP decreases in columnar zone and coarse-grained reheated zone (CGRZ); the volume fraction and the size of nonmetallic inclusions in weld metal cannot be affected by the change in Cu and Cr contents, and the diameters of most inclusions are in the range of 0.1–1.0 μm; tensile strength of weld metal increase because of solid solution hardening effects of Cu and Cr; impact toughness decreases with the increase of Cu content, but increases with the increase of Cr content.
Beidokhti et al.19) studied the influences of Ti and Mn on HSLA submerged arc weld metal properties. Weld metal in 1.92% Mn-0.02% Ti and 1.40% Mn–0.08% Ti has optimal mechanical properties. The AF in weld metals increases with an increase of Ti content in the range of 0.02–0.08% as shown in Fig. 7.19) Mn may refine and homogenize the weld microstructures. Further addition of Ti or Mn encourages grain boundary nucleation frequency of bainite with higher than intragranular nucleation of AF and thus weld metal hardness increases. Best combination of microstructure and impact properties can be obtained in the range of 0.02–0.05% Ti in weld metal. Further addition of Ti can accelerate weld metal microstructure change from the mixture of AF, GBF, and Widmanstatten ferrite (WF) to a mixture of AF, GBF, bainite and ferrite with M/A.20)
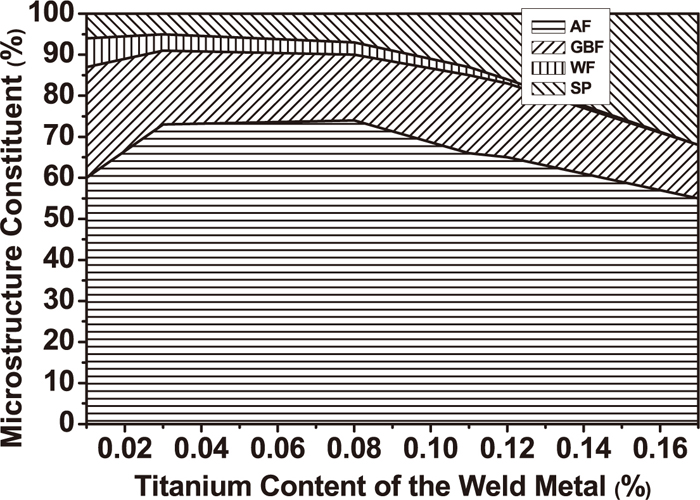
Effect of Ti content on weld metal microconstituents.19)
By changing the Al/O ratio from 0.48 to 1.52, the inclusion nucleation core in a low alloy steel weld pool (main reaction of deoxidation process) was observed by Terasaki et al.21) The results showed that with low aluminum contents, the glassy phase in Mn–Al–Si–O system dominated the inclusion core, while with middle aluminum contents, corundum alumina and some glassy phase serve as the inclusion core. Kojima et al.22) studied the case where the Al/O ratio is low (0.2 and 0.43) in weld metal. The experiment showed that when the Al/O ratio is lower than 0.45, a reasonably high Al/O ratio is favorable for the toughness mainly because there are more 0.2–0.8 μm inclusions and AF nucleation is accelerated.
Yamada et al.23) used an ion beam to make a layer of Ti-enriched film on inclusion surface with three Al/O ratios to investigate the relationship between the Ti-enriched layer and AF in low carbon Ti–B weld metals. (1) In samples with the Al/O ratio of 0.48 and 0.73, inclusions near AF formation are surrounded by narrow TiO layers with its thickness in the range of 10–40 nm; (2) the TiO layer has a B–N orientation relationship with AF; (3) the TiO layer on the inclusion surface contributes to the heterogenous nucleation of AF.
The influence of S on AF is reflected in sulfide inclusion. Liu8) studied the relationship between AF nucleation and sulfide inclusion without special alloying elements. (1) Complex Mn–Fe–S–O oxides and relatively pure SiO2 have no effect on AF nucleation. But iron sulfide particles including a little concentration of Mn and Cu are effective for AF nucleation. (2) CuxS particles have no effect on AF nucleation. (3) Mn-depleted zone and P-rich zone around iron sulfide inclusion in the iron matrix may be the two reasons that iron sulfide can nucleate AF. It is concluded by Sarma et al.11) that there is little difference in thermal expansion coefficients between austenite and MnS. The possibility of particles covered by MnS as AF nucleation is lower than that of Ti-oxide inclusions. Precipitated MnS layer on the surface of Ti-oxide inclusions may decrease the possibility of AF nucleation on inclusions.
Terashima et al.24) studied the effect of oxygen concentration of weld metals on toughness as shown in Fig. 8. Oxides may accelerate AF formation, the toughness of high strength steel weld metal is the highest at 20 ppmw O, and decreased as an increase of O concentration in the range of 20–100 ppmw, and afterwards there is a peak value of the toughness at 300 ppmw. Figure 9 shows effect of oxygen concentration in high strength weld metal on continuous cooling transformation (CCT) diagrams.24) The increase of oxygen concentration may enlarge bainite and ferrite areas because oxide may provide extra heterogeneous nucleation sites.
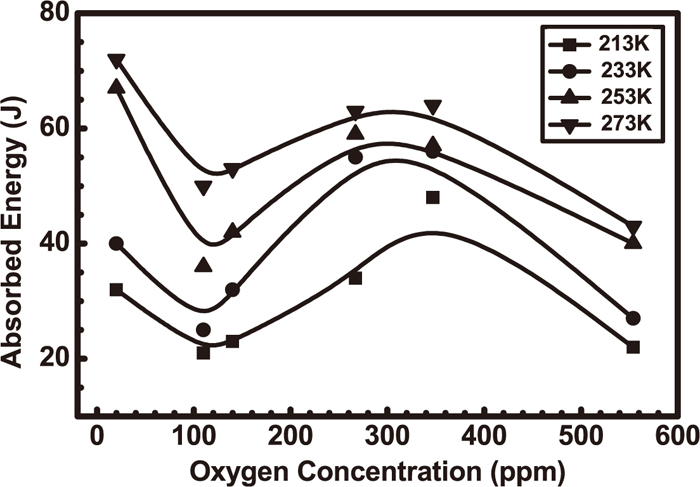
Effect of oxygen concentration on absorbed energy of HSLA weld metal.24)
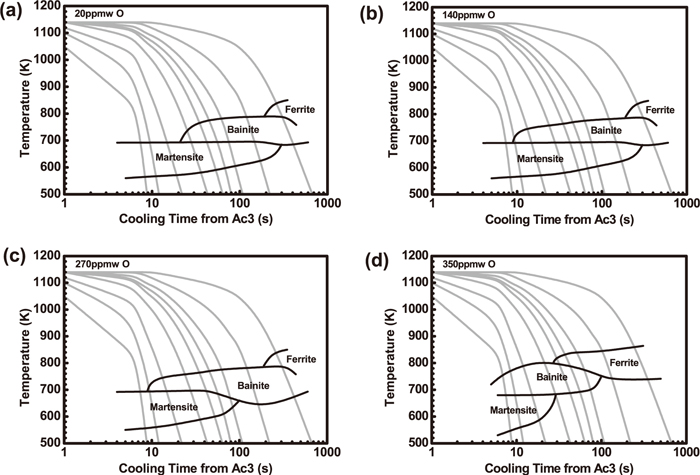
CCT diagrams for HSLA weld metal.24) (a) 20 ppmw O; (b) 140 ppmw O; (c) 270 ppmw O; (d) 350 ppmw O.
Lee et al.25) researched the effect of B on AF nucleation with three experimental FCWs with B content of 32 ppmw, 60 ppmw, 103 ppmw in weld metals. The volume fraction of AF decreases with an increase of B in the range of 32–103 ppmw. When B content is 103 ppmw, upper bainite instead of AF is formed. The impact energy of weld metals slightly decreases with an increase of B content in the range of 32–60 ppmw, but significantly decreases with an increase of B content in the range of 60–103 ppmw, duo to B causes a reduction of eutectoid temperature. Warren et al.26) studied the effect of lanthanum on toughness in ultra-high strength steels. Higher toughness can be obtained by the addition of 0.015% lanthanum in MnS inclusions in AF1410 ultra-high strength steels.
2.1.3. Effect of Inclusions on AF NucleationThe size, quantity, composition, metallurgy of inclusions and other factors have great effects on AF nucleation in weld metals. AF nucleation occurs in weld cooling process.27) Energy barrier to heterogeneous nucleation of ferrite at inclusions decreases with an increase in the inclusion diameter from 0 to 1 μm because of an increase in the inclusion particle surface area. When the inclusion diameter is larger than 1 μm, the energy barrier value slightly decreases with the further increase in inclusion size. Therefore, it is not necessary to further increase the inclusion diameter to promote ferrite nucleation on an inclusion surface. The critical value of an inclusion particle diameter for AF heterogeneous nucleation is 1 μm.11) Grong28) also thought that inclusions of about 1 μm size could promote AF nucleation without harmful effect for mechanical properties duo to relatively small size.
Nevertheless, Ramirez14) proposed that the volume fractions of nonmetallic inclusions in weld metals are mostly in the range of 0.2–0.6%, and rarely in the range of 0.8–1.1%. The inclusion density ranges from 1.2×108 to 5.4×108 mm–3, and the average and maximum value of the inclusion diameter are respectively in the ranges of 0.3–0.6 μm and 0.9–1.7 μm. Inclusion particle size distribution is related to flux basicity. Average inclusion size does not drastically change with an increase in O and S content, but inclusion average size increases when O plus S content increases to above 400 ppmw. Inclusions as AF nucleation are mostly within 0.2–0.6 μm in diameter and are chemical heterogeneous compounds containing various elements. Inclusions reduce the energy barrier as high energy inert substrates to promote AF nucleation. When AF nucleates with inclusion as the core, abundant interlaced AF nucleation may be caused.29) Lee et al.13) found that it was easier to get nucleation in large size inclusions than in small ones as shown in Fig. 10. It can be seen that the nucleation probability is near to zero when the size of the inclusions is less than 200 nm; the probability for AF nucleation increases markedly when inclusion diameter increases in the range of 0.4–0.8 μm, and the value is 1.0 at 1.1 μm; inclusions with a diameter larger than 1 μm have greater possibility for AF nucleation.
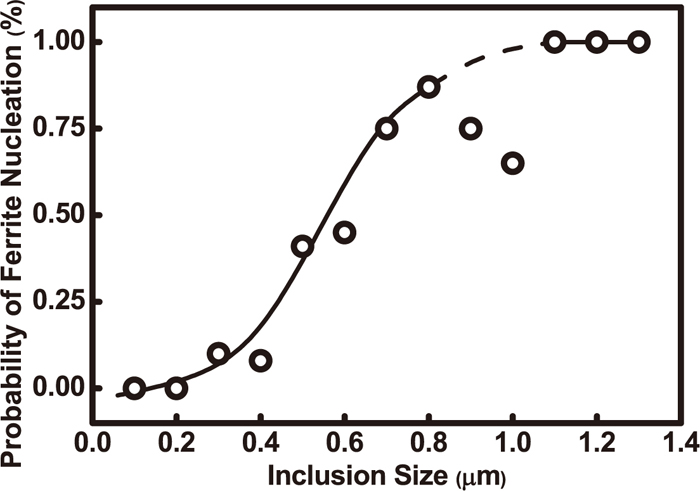
Effect of inclusion size on the probability of ferrite nucleation.13)
AF in weld metal is proportional to the number density of inclusion with a diameter smaller than 2 μm.30) The density of fine inclusions with their diameters in the range of 0.2–0.6 μm may be increased by electromagnetic stirring in welding and the number of AF increases.31) Figure 11 shows the total size distribution of all inclusions and inclusions for AF nucleation.13) It can be seen that AF nucleation has the largest quantity with an inclusion diameter in the range of 0.5–0.8 μm, which has large influence on the formation of microstructure with a high ratio of AF.
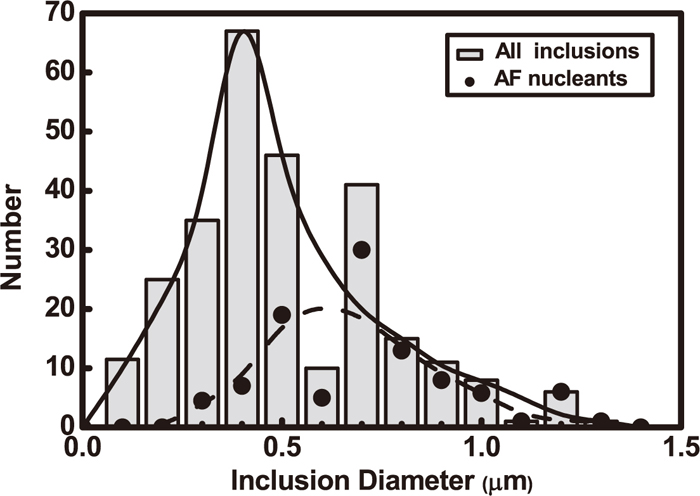
Inclusion distribution diagram in weld metal of mild steel.13)
Al2O3 in oxide form is not effective for AF nucleation. On the contrary, it may accelerate bainite formation. But MnO–Al2O3 is favorable for AF nucleation. Hidaka32) studied the influence of oxides on microstructure and impact toughness of high strength steel weld metals and concluded that FATT of weld metals is related to the size of intragranular bainite nucleated by oxide inclusions. FATT is also influenced by the density of dispersive oxide inclusions and the impact toughness can be improved as the refined microstructure with Ti–Mn oxide. Oxygen content in weld metals made by TiO2 type FCW may be reduced as a decrease of TiO2 activity. Ti-oxide is the best particle for AF formation. There is low mismatch and a similar orientation relationship between TiO and α, so AF can be formed. Ti-enriched inclusions can accelerate the kinetics of AF formation in weld metals. Inclusions have various shapes and textures in weld metals including spherical, faceted and agglomerations. The inclusion core is mainly comprised of oxides like Ti, Mn, Si and Al in different proportions and performs as a complex deoxidation product.14)
In order to obtain more effective AF, it is necessary to study the inclusion metallurgy from the aspect of welding metallurgy. Andersson et al.33) proposed two points: one is to obtain microstructures with optimum mechanical properties from the aspect of inclusion engineering; the other is to produce cleanliness steel and then obtain AF microstructure by the addition of active inclusions separately. Zhang et al.34) studied the BaF2–Al–Mg slag system of high toughness all-position self-shielded FCW. By the addition of 10% Fe2O3 and 5% MnO2 in the flux, the Al content can be reduced to 0.84% and oxygen content be increased to 85 × 10–6 in deposited metal, avoiding the influence of δ-ferrite and aging effect. Circular inclusions of Al2O3 as its main constituent are produced in weld metals and intragranular heterogeneous nucleation particles are increased. Main weld microstructures with AF are formed and therefore the low temperature toughness of deposited metals is improved significantly.
It should be pointed out that it is difficult to decide which mechanism that is most important for AF nucleation. Instead, it is believed that the four mechanisms often work together to promote the AF nucleation on inclusions. For promoting the AF formation, it is necessary to optimize alloying elements (see Table 3). Base on the influence on AF nucleation, inclusions can be classified into active and inert. The effect of two kinds of inclusions on AF nucleation is shown in Table 4.11) It can be seen that active inclusions for AF nucleation are mostly complex and multi-phase inclusions. The critical value of an inclusion diameter for AF nucleation is 1 μm.
| Element type | Alloying elements | Optimum range/% | Influence on AF | Welding process |
|---|---|---|---|---|
| Increase austenite zone | C | 0.05–0.15 | Small increase | SMAW |
| Mn | 1.40–1.92 | Mild increase | SAW | |
| Ni | 2.03–2.91 | Moderate increase | SAW | |
| Cu | 0.14–0.94 | Moderate increase | SMAW | |
| Increase ferrite zone | Mo | 0.7–0.995 | Large increase | SAW |
| Cr | 0.05–0.91 | Moderate increase | SMAW | |
| Ti | 0.02–0.05 | Moderate increase | SAW | |
| B | 0.003–0.006 | Small decrease | FCAW |
| Compound added | Active inclusions for AF nucleation | Inert inclusions for AF nucleation |
|---|---|---|
| Simple oxides | Ti-oxides (Ti2O3 and TiO) | Al2O3, SiO2, Ti2O3 |
| Complex oxides | (Ti, Mn)2O3, TiO2–(MnO–Al2O3) | MnO–SiO2, MnO–FeOx–SiO2, MgO–Al2O3 MnO–Al2O3 |
| Galaxite spinel MnO–Al2O3 | ||
| Simple nitrides | TiN, VN | TiN |
| Simple sulfides | MnS, CuS | |
| Complex oxy-sulfides and multi-phase inclusions | Al2O3–MnS, TiO2–Al2O3–MnS Ti- and Ti-Ca-oxy-sulfides Ti2O3–TiN–MnS, TiOx–TiN–MnS FeS–(Mn,Cu)S, MnS–VC, MnS–V(C, N) |
The current recommended control level of S and P for cleanliness steel is less than 0.005% and 0.015% respectively. The actual content of S and P is mostly less than 0.003% and 0.009%, and that of weld metals is usually in the range of 0.008–0.014% and 0.012–0.014% respectively. S and P mainly influence the toughness and crack resistance of weld metals. N and O contents in steels have been less than 250 ppmw, while that in weld metals is usually in the range of 300–900 ppmw. Therefore, desulfuration, dephosphorization and denitrification are quite important for HSLA steel weld metals.
2.2.1. Dephosphorization of Weld MetalsP has no obvious effect on weld microstructures, but it can increase the hardness and strength of weld metals and reduce impact toughness and crack resistance. When P content in weld metals is less than 0.005%, further reduction of its content has little influence on its properties. The mechanism of dephosphorization in weld metals is mainly to form phosphate. Dephosphorization can be done by the metallurgical reaction and there is a limit which is related to basicity of flux and contents of P in the wire and base metal. The limit value of P in weld metal is about 0.010 ± 0.002% for high basicity fluxes of B1 ≥ 2.5.35)
Thermodynamic calculation and experiments in ferrous metallurgy show that it is possible to adopt oxidative dephosphorization and reductive dephosphorization in weld metal. But for most welding consumables, FeO in large quantities is not allowed in basic slag, and CaO in acid slag is not allowed to have greater activity. Therefore, dephosphorization becomes a relatively difficult process.
Reductive dephosphorization must be realized by the addition of a deoxidant that is better than Al, so that molten steels can reach deep reduction. Table 5 shows the properties of various phosphides.2) It can be seen that P and alkali earth metals such as Ca, Mg, Ba can produce more stable chemical compounds with a lower density than Fe3P and Fe2P, which proves that dephosphorization is possible under reductive conditions. In the study of oxidative dephosphorization, it is shown that the affinity of O and Si is greater than that of O and P.
| Phosphide | P2O5 | Ca3P2 | Mg3P2 | Ba3P2 | AlP | Fe3P | Fe2P | Mn3P | Na3P2 |
|---|---|---|---|---|---|---|---|---|---|
| Valence | +5 | –3 | –3 | –3 | –3 | – | – | – | –3 |
| –ΔfHθm (298 K)/KJ mol–1 | 1492 | 506 | 464 | 494 | 164.4 | 164 | 160 | 130 | 133.9 |
| Density/g cm–3 | 2.39 | 2.51 | 2.06 | 3.18 | 2.42 | 6.80 | – | 6.77 | 1.74 |
| Melting point/°C | 580 | 1320 | – | 3080 | – | 1220 | 1370 | 1327 | – |
By the addition of calcium dephosphorization flux in CaO–CaF2 slag, effect of precipitation dephosphorization in Mn–Si–Fe alloy was investigated. The study concentrates on the influence of dephosphorization flux content and increment of Si content in Mn–Si–Fe alloy on dephosphorization. When the dephosphorization flux quantity increases from 2.5% to 7.5%, the dephosphorization effect increases 5.56%. When the Si content in the alloy increases from 19% to 25%, the dephosphorization effect may increase by at least 23.22%. This shows that an increase in Si content in Mn–Si system has a much greater influence on the dephosphorization effect than the addition of dephosphorization flux only. The increase in Si content can reduce oxygen potential, and can also improve P activity so that Ca in dephosphorization flux enters into the slag in the form of Ca2P3 rather than CaO.36)
It is shown that the addition of 3% Si–Ca alloy to ultra-low hydrogen high toughness stick electrodes can reduce the mass fraction of P content in weld metals from 0.015% to 0.008%, which proves that Si–Ca alloy does play a role in reducing dephosphorization. Rare earths, as good reducing agent in molten steel, have strong chemical activity. They can reduce P in molten steel to P3– to reduce dephosphorization of molten steel.2) O and S contents in molten steel are the main factors that influence reducing dephosphorization using rare earths. In order to improve rare earth dephosphorization efficiency, it is crucial to control O content in molten steel to be in about 1% and 0.010% respectively during reductive period.37)
BaO slag systems can improve the dephosphorization rate in an atmosphere of low oxygen. In recent years, BaO dephosphorization flux has been adopted in the hot metal extra furnace dephosphorization process, so that the dephosphorization rate in low oxygen atmosphere has been improved. BaO can enter into slag by adding 10–20% barium carbonate to a CaO slag system after the high temperature decomposition of barium carbonate. This greatly reduces P2O5 activity in slag so that the dephosphorization effect is notably improved. In electrodes with high strength and toughness, replacing CaCO3 with BaCO3 not only reduces S and P content in weld metals, but also improves the operative performance.2)
2.2.2. Desulfuration of Weld MetalsRecently, deep desulfuration for molten steel has proceeded to improve the desulfuration effect in steel making. One way to accomplish this is to by using a Mg matrix, Ca and Ba and its alloy desulfuration flux to perform deep desulfuration for molten steel. At the same time, O and P content is reduced. Another way is using previously frequently adopted basic oxides like CaO, MgO and MnO during the process of basic slag desulfuration. But recent research focuses on the influence of the addition of Li2O and BaO in refining slag on the desulfuration effect in the external refining deep desulfuration treatment. When more than 7.5% Li2O is added, the desulfuration rate is higher than 90%, and when the addition reaches 20%, the desulfuration rate is higher than 95% with the final S content in molten steel reducing to less than 0.002%. Another study shows that in the CaO–Al2O3 refining slag system, when the ratio of CaO to Al2O3 is in the range of 2.5–3.0, optimum desulfuration can be obtained by the addition of 10–13% BaO. S content in molten steel reduces from 0.011% to 0.0028% after refining.2) The above mentioned technological approaches for improving the desulfuration effect can be adopted in the study of welding metallurgy and design of welding consumable.
In addition, rare earth with a strong desulfuration capacity can interact with S to form high melting sulfide. By addition of rare earths to high Mn steel, rare earth sulfides (RES, RE2S3) and rare earth sulfur oxides (RE2O2S) are formed, which are of 2000°C melting point and dispersed in intracrystalline as fine particles. The addition of a rare earth reduces the quantity of sulfide inclusions, and most importantly the addition can improve shape (circular shape granular), size (fine), distribution (change from segregation in grain boundary and intragranular to dispersion in intragranular) and greatly reduce the deleterious effect of nonmetallic inclusions in high Mn steel. So, the influence of rare earth on the desulfuration effect is greatly related to reducing slag. When reducing slag dioxide is favorable, the desulfuration rate of rare earths is on average higher than 52%; when reducing slag dioxide is not favorable, the desulfuration rate of rare earths is on average about 20%. S content in weld metals can be controlled to 0.001% by Ba, Mg and a basic slag system. But the problem, that is an acid slag system with a good operative performance, has not yet achieved this level.37)
2.2.3. Denitrification of Weld MetalsWhen N content in weld metals is higher than 0.01%, the impact absorbed energy of weld reduces sharply. Therefore, this is one of the important measures to improve weld metal toughness by reducing N content. To reduce the deleterious effect of N, one way is by improving smelting technology to a lower N content in welding materials; another way is by the addition of microalloy elements (e.g. Elements formed by nitrides like Al, Ti) to produce nitrides and then to reduce the deleterious effect of free N.
Various new complex ferroalloys may play a part in comprehensive metallurgical processes such as deoxidation, denitrification, desulfuration and dephosphorization. Alkaline earth metals such as Ca and Ba have a notable effect on deoxidation, dephosphorization and desulfuration in the metallurgical process. From thermodynamic calculations, the deoxidizing capacity of Ca and Ba is much greater than that of Al. but in practical applications, the actual deoxidizing capacity may barely reach the thermodynamic calculation value because the solubility of Ca and Ba in molten steel is affected by many factors and their vapour pressure is quite high. There has been much research and discussion on finding a solution to this problem. It has been found that addition of Ba and Ca into molten steel through a complex ferroalloy can reduce loss, increase the solubility of Ba and Ca in molten steel, and improve their metallurgical capacity. In a multicomponent alloy containing Ba, the deoxidizing capacity of Si may be equal to that of Al, the deoxidization, desulfuration and dephosphorization capacity of Ba may be fully demonstrated and Al and Ti can play a part in denitrification and nitrogen fixation. So it is necessary to discuss a way to further deoxidization and denitrification by the proper adoption of alkaline earth metals and multi-element complex ferroalloys.
2.3. Crack Resistance of Weld Metal 2.3.1. Cold Crack ResistanceAs welding structural steel develops towards a low carbon equivalent and high strength, hydrogen-induced cold cracking position in welding joint has transferred from HAZ to weld metal. Cold crack resistance improvement is realized mainly through controlling the microstructure, impurity content, optimizing weld performance, reducing diffusible hydrogen and so on.
Adding fluorides such as CaF2, Na3AlF6, K2SiF6, MnF3 and MgF2 to rutile-type FCWs at concentrations of 1.8–2.3% can reduce hydrogen content in weld metals. The effect of the fluoride content on diffusible hydrogen is shown in Table 6.38) It shows that all fluorides can reduce diffusible hydrogen content in weld metals, but with a varying reduction range. Wires with CaF2 have the lowest diffusible hydrogen content (6.32 mL (100 g)–1), while wires with MnF3 have the highest content (7.96 mL (100 g)–1). The diffusible hydrogen content in weld metals decreases with an increase in the slag basicity. Wires with CaF2 and highest basicity (0.27) have the lowest diffusible hydrogen content, while wires with MnF3 and the lowest basicity (0.01) have the highest diffusible hydrogen content. Thus, diffusible hydrogen content in the weld metals is influenced greatly by slag basicity rather than by fluorides.
| Wire | TiO2 | Mn | Fe | Ni | Fluoride | HDMa/mL (100 g)–1 | HFMb/mL (100 g)–1 | ||
|---|---|---|---|---|---|---|---|---|---|
| Discrete values | Avg. | Discrete values | Avg. | ||||||
| A | 37.0 | 10.5 | 4.5 | 9.5 | CaF2 2.3 | 7.01, 6.31, 5.98, 5.97 | 6.32 | 3.55, 3.26, 3.27, 3.20 | 3.32 |
| B | 37.0 | 10.5 | 4.7 | 9.5 | Na3AlF6 2.1 | 7.92, 7.59, 7.29, 6.80 | 7.40 | 4.31, 4.34, 4.20, 3.94 | 4.20 |
| C | 37.0 | 10.5 | 4.6 | 9.5 | K2SiF6 2.2 | 8.29, 7.38, 7.07, 7.07 | 7.45 | 4.26, 4.26, 3.90, 4.28 | 4.18 |
| D | 37.0 | 10.5 | 4.6 | 9.5 | MnF3 2.2 | 8.56, 8.56, 7.44, 7.28 | 7.96 | 4.52, 4.87, 4.15, 3.98 | 4.38 |
| E | 37.0 | 10.5 | 5.0 | 9.5 | MgF2 1.8 | 7.74, 7.98, 6.37, 6.40 | 7.12 | 4.18, 4.31, 3.56, 3.61 | 3.92 |
| F | 37.0 | 10.5 | 6.8 | 9.5 | 0 | 10.68, 10.15, 9.94, 9.89 | 10.17 | 7.64, 7.30, 6.70, 6.86 | 7.13 |
a HDM stands for diffusible hydrogen content in deposited metal, b HFM stands for diffusible hydrogen content in fused metal
Brown39) studied the reason for centerline cold cracking in weld metal caused by microsegregation of Mn, Ni, and Si when using flux cored wire to weld API 5L-X80 steel. The results show that the crack path is perpendicular to the initially formed δ-ferrite cellular dendrite and growth direction of the intercellular-dendritic segregation formed in δ -ferrite cellular dendrites. Micro-segregated regions are rich in hardening elements, which results in a higher hardness in the segregation region. The increased hardness value is related to a reduction in toughness. Weld metal is sensitive to cracks in micro-segregated regions. Beidokhti et al.40) studied the effects of different Ti content on hydrogen-induced cracking, hydrogen sulfide stress cracking of the submerged arc welded API 5L-X70 steel weld metals. Increase of AF content in the weld metal can improve hydrogen-induced cracking resistance and hydrogen sulfide stress cracking capacity. More than 30% bainite and M/A in weld structures may cause testing failure of hydrogen-induced cracking resistance and hydrogen sulfide stress cracking capacity. The addition of Ti to welds may change the nature of inclusions from Mn-based inclusions to Ti-based ones. The precipitated titanium carbonitrides may delay cracking in H2S environments as beneficial hydrogen traps. Through further addition of Ti, bainite and M/A weld metal structures appear and outweigh the beneficial effect of titanium carbonitrides. As a result, weld metals with a high percentage of AF and good distribution of titanium carbonitrides show the best performance in acid medium. Jin et al.41) studied the effect of nonmetallic inclusions on hydrogen-induced cracking of API 5L-X100 steel. It is showed that API 5L-X100 steel contains inclusions such as elongated MnS inclusions and spherical Al-, Si-, Ca-Al-O-S-enriched inclusions. Most inclusions in steels are Al-enriched. Cracking is mainly related to Al- and Si-inclusions and has no relation with elongated MnS inclusion. The limit value of H content for hydrogen-induced cracking in API 5L-X100 steel is 3.24 ppmw.
With improvements in the performance of steels and welding consumable, many past testing methods for the cracking evaluation of base metal and weld metal are now out of date. So it is important to improve the testing methods for cold cracking sensibility of weld metals. Using the original Y groove cracking test, the joint does not crack even at –10°C, which is not helpful for the estimation of the crack resistance of weld materials. Based on the Y groove type cracking test, Authors improved Y groove crack test by changing the groove angle and enlarging the gap and test plate thickness. In this test, the welding current was 250 A, arc voltage was 29 V, and the weld heat input was 1.6 KJ mm–1. The whole experiment was conducted at 0°C. The experimental design and results are shown in Table 7. The cold crack resistance of an E71-T1 FCW welding joint was studied. The cracking formation rate is the highest with the groove angle being 120°. Cracking formation rate increases with an increase in groove angle. The cracking formation rate may increases by enlarging the gap and plate thickness. In addition, a crack can also form by further increase of restraint intensity and stress.
| Type | Groove angle | 60° | 75° | 90° | 105° | 120° |
|---|---|---|---|---|---|---|
| Gap | 2 mm | 33 | 35.5 | 41.3 | 52.5 | 64.3 |
| 3 mm | 42.5 | 43.5 | 15.1 | 55.2 | 72.3 | |
| 4 mm | 63.3 | 72.3 | 100 | 100 | 100 | |
| Thickness | 20 mm | 33 | 35.5 | 41.3 | 52.5 | 64.3 |
| 25 mm | 37.8 | 45.5 | 45.5 | 53.5 | 71.2 | |
| 30 mm | 39.2 | 55.5 | 51.5 | 55.5 | 77.7 |
By using the G-BOP test, Zhang et al.42) studied the effects of welding current, CO2 shielding gas flow and diffusible hydrogen content on the weld metal cold crack sensibility of three kinds of E71T-1 FCWs. With an increase in weld current and a reduction in CO2 shielding gas flow, the diffusible hydrogen content in the FCW weld increased. At room temperature of (25±3)°C, H content for the cold crack appearing in the FCW in the G-BOP test was about 6 mL (100 g)–1. Authors also conducted the experiments to modify the G-BOP test by enlarging welding restraint intensity. The cold cracking rate can be as high as 63%. These improvements in the traditional testing methods are helpful for estimating modern welding consumable crack resistance.
By employing modern production procedure, the diffusible hydrogen content in acid FCW is less than 5 mL (100 g)–1. But seeking a very low level of hydrogen should not be the goal. Accurate and rapid detection of diffusible hydrogen in weld metals is very important. There are certain error in the chromatography, glycerol, hot extraction and mercury method. When diffusible hydrogen content is in the range of 5–10 mL (100 g)–1, the measurement error can reach ±1.5 mL (100 g)–1. When the diffusible hydrogen content is less than 5 mL (100 g)–1, the measurement error can reach ±1 mL (100 g)–1. The welding voltage, current, stick out, weld feeding speed, test temperature, test time, environment humidity and deposited metal quantity have great influence on the testing result of diffusible hydrogen. Therefore it is difficult to meet the requirement of low hydrogen and super low hydrogen for testing accuracy. Currently the nominal diffusible hydrogen content of seamless FCW has been less than 3 mL (100 g)–1. The actual diffusible hydrogen of low hydrogen stick electrodes for high strength steel has been 1.8–2.2 mL (100 g)–1.
2.3.2. Hot Crack Resistance of Weld MetalsIto et al.43) developed a high quality FCW for shipbuilding and bridge construction, and the FCW have excellent hot crack resistance in small gap groove welding. Table 8 shows the range of chemical composition of hot crack resistance and the chemical composition of the FCW.43) Figure 12 shows the effect of P, Mn and C content change on a hot crack resistance.43) It is clear that a reasonable control of Mn and C content, especially the reduction of P content can increase the crack resistance. When P content is in the range of 0.009–0.012%, Mn is 1.05–1.10% and C is 0.007–0.008%, the crack formation rate is the lowest. P can reduce the final solidification temperature of liquid residues in the interdendritic area and thus increases the crack sensibility of weld metals. S may intensify the deleterious effect of P.44)
| Element | Optimum range/wt.% | Experimental/wt.% | Conventional/wt.% | |
|---|---|---|---|---|
| Metals | C | 0.07–0.09 | 0.08 | 0.07 |
| Si | 0.40–0.50 | 0.50 | 0.60 | |
| Mn | 1.25–1.60 | 1.35 | 1.10 | |
| Impurities | P | Low | 0.010 | 0.017 |
| S | Low | 0.009 | 0.012 | |
| Others | Al | – | 0.020 | 0.010 |
| Ti | – | 0.040 | 0.035 |
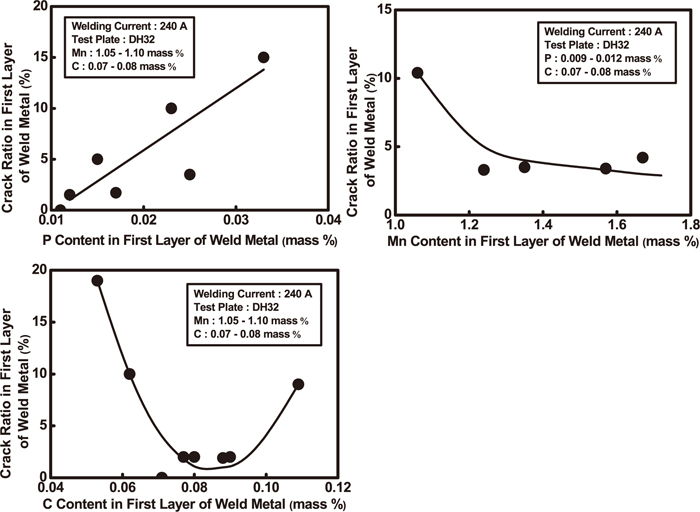
Effects of P, Mn and C contents on hot crack resistance.43)
By increasing the plate gap, slot of Paton cracking test, the effects of different welding parameters and FCWs on hot crack with a ceramic backing were investigated in different restraint intensity. The most important measure to prevent the backing crack is that the welding current should be within 200 A and the welding speed should be within 150 mm min–1. A newly-developed FCW can increase the current from 200 A to 260 A and the welding speed from 150 mm min–1 to 200 mm min–1 in the backing weld. At the same time, when welding with ceramic backing, the side near the ceramic backing would have different metallurgical reaction conditions from the side far from the ceramic backing. For the former one, there is the problem of the reduction of the silicon oxide, which may increase oxygen and silicon in weld metals. Therefore, the side near the ceramic backing has more silicon content than that distant to the ceramic backing. The decrease in Si content may cause a lower toughness.45)
2.4. The Fumes and the Environmental-friendliness of Welding ConsumablesCurrently the mechanisms of welding fumes and their effects on the human body are key aspects of a new generation of welding consumables. Thus, it is a key issue in the research of a new generation welding consumables.
Chu et al.46) centered on TiO2–CaO–SiO2–CaF2 slag systems and studied on the effect of flux slag system and alloy systems on weld fume quantity and operative performance. It considers that based on the welding performance, an appropriate increase in TiO2 and iron content, a reduction in CaF2 content, and increase in calcium alloy can lower the quantity of electrode fume. The quantity of fume in this newly-developed electrode is 9.13 g Kg–1 and its welding performance is favorable. The influence of the addition of the iron powder content on the fume formation rate (FFR) is related to the slag system of FCW. Under the current slag system, FFR will reduce as the iron power content decreases. Li47) studied on the effect of the production process of the iron powder on FFR. The results show that FFR of the spherical atomization iron powder is slightly higher than that of the erose deoxidized iron powder. Different FFRs are caused by different production processes of iron powder and different amounts of iron oxide on the surface of iron powder. The quantity of iron oxide on the surface of spherical atomization iron powder is greater than that of erose deoxidization iron powder. During the welding process, iron oxide evaporated very quickly so that the iron oxide in the fumes increased, and FFR also increased. Further, the form of the iron powder determined the conductivity and apparent density of the powder. The size of the electric conduction section of the wires would be affected and thereby the arc density and arc temperature distribution would also be affected. But this influence was limited. The effects of size distribution of atomization and deoxidization iron powders on FFR are shown in Fig. 13.47)
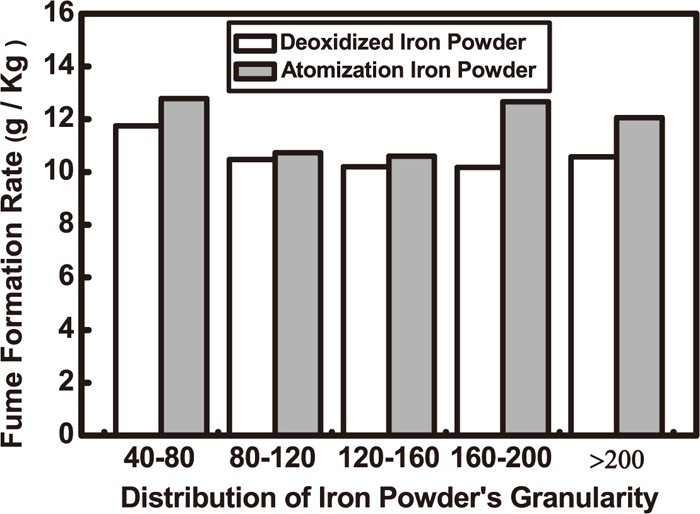
Effect of iron powder’ granularity distribution on the FFR.47)
The effect of mineral powder content on FFR is shown in Fig. 14.48) The FFR of FCWs increases with an increase in sodium fluosilicate content. A favorable match of feldspar and alloy can reduce the FFR of FCWs. FFR decreases from 8–12 g Kg–1 to 4–4.5 g Kg–1 with an increase in feldspar content. An additional amount of ilmenite has little influence on the FFR of FCWs, while the stability (especially its high temperature stability) of mineral powder has a great influence on the FFR. The effect of powder pretreatment on the FFR differs: smelting powder can reduce the FFR, while the powder granulation influence is not obvious. A reasonable match of mineral powders can lower the FFR of FCWs.
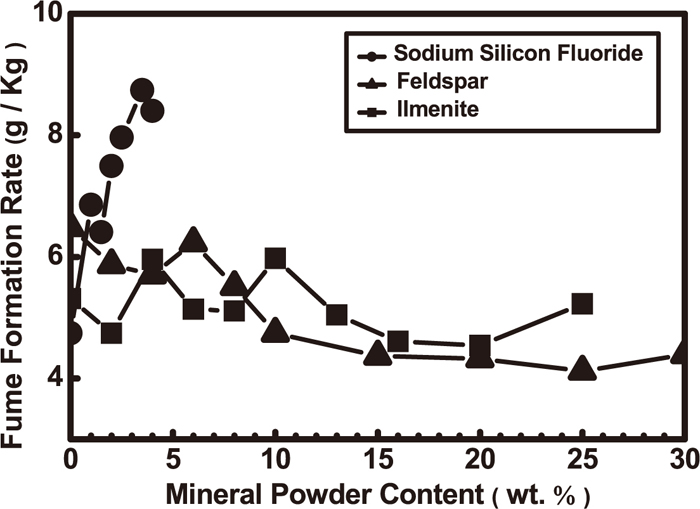
Effect of mineral powder on the FFR of FCW.48)
The effects of shielding gas on the FFR and the fume particles are shown in Tables 9 and 10.48,49) The FFR changes significantly with an increase of CO2 in the shielding gas. Fume particle size becomes larger with an increase of O2 and CO2 in the shielding gas except the components of Ar-12% CO2-4% O2. This is due to O2 and CO2 increasing, which would make nucleation with a higher driving force prompt particles to form nucleation at high temperatures that would be benefit the formation of coarse particles. When the diameter is less than 100 nm, the shielding gas composition has little effect on the form of fume particles; binary Ar–CO2 that adds 2% O2 has no effect on the FFR. When oxygen is added into the binary mixed gas, the FFR is only larger when 5% CO2 is added. Increasing He or CO2 into a ternary Ar–He–CO2 mixtures has little effect on the FFR or particle size distribution. The majority of fume particles are spherical single crystals whose mainly composition is (Fe, Mn)3O4. In the Ar-based shielding gas, an increasing in the FFR is closely related to increases in CO2 which can be attributed to the CO2 affecting metal transfer and arc characteristics. Increasing CO2 content will form droplets with a larger size, longer separation time, higher arc temperature, which in turn will increase the production of fume.48,49)
| Gas composition | FFR/g min–1 | O2 index/% | O/wt.% | Si/wt.% | Mn/wt.% | Fe/wt.% | Mn/Fe |
|---|---|---|---|---|---|---|---|
| Ar-5%O2 | 0.274 | 5 | 27.5 | 0.9 | 8.7 | 62.8 | 0.14 |
| Ar-5%CO2 | 0.246 | 2.5 | 27.5 | 0.7 | 7.0 | 64.8 | 0.11 |
| Ar-10%CO2 | 0.298 | 5 | 27.4 | 0.3 | 5.9 | 66.4 | 0.09 |
| Ar-18%CO2 | 0.396 | 9 | 28.1 | 1.3 | 4.2 | 66.3 | 0.06 |
| Ar-5%CO2-2%O2 | 0.242 | 4.5 | 27.5 | 0.6 | 7.4 | 64.5 | 0.12 |
| Ar-12%CO2-2%O2 | 0.312 | 8 | 27.8 | 1.0 | 5.8 | 65.3 | 0.09 |
| Ar-18%CO2-2%O2 | 0.392 | 11 | 28.4 | 2.3 | 7.0 | 62.3 | 0.12 |
| Ar-5%CO2-5%O2 | 0.352 | 7.5 | 28.1 | 1.6 | 6.1 | 64.2 | 0.10 |
| Ar-12%CO2-4%O2 | 0.318 | 10 | 28.1 | 1.6 | 6.1 | 64.2 | 0.10 |
| Ar-20%He-12%CO2 | 0.279 | – | 28.1 | 1.3 | 4.0 | 66.6 | 0.06 |
| Ar-30%He-6%CO2 | 0.273 | – | 27.7 | 0.8 | 6.1 | 65.4 | 0.10 |
| Ar-30%He-10%CO2 | 0.277 | – | 27.7 | 0.8 | 4.8 | 66.8 | 0.07 |
| Particle size range | O/wt.% | Si/wt.% | Mn/wt.% | Fe/wt.% | Mn/Fe |
|---|---|---|---|---|---|
| < 20 nm | 28.4 | 2.1 | 6.8 | 62.8 | 0.11 |
| 21–40 nm | 28.3 | 2.1 | 6.7 | 62.9 | 0.11 |
| 41–60 nm | 27.8 | 1.2 | 7.0 | 64.1 | 0.11 |
| 61–80 nm | 27.6 | 0.7 | 6.4 | 65.3 | 0.10 |
| > 81 nm | 27.5 | 0.4 | 5.4 | 66.8 | 0.08 |
Welding technology, including welding conditions and polarity, exerts an effect on the diffusion rate of the welding consumables. Increasing hot input also makes FFR increase.50) The energy input influences not only the FFR, but also the composition and structure of the smoke and the gas. The FFR of FCW is influenced by factors including droplet transfer mode, arc stability, shielding gas composition and welding spatter and so on. Granular transfer causes high FFR, and spraying transfer can somehow decrease the FFR. The FFR increases as the shield gas oxidation property increases; the increase in shield gas oxidation property will increase spatter which will also heighten the FFR.51) The latest research proves that in the welding fume of the FCW, slight spatter takes 30% of the entire fume, which also corresponds to respirable particulate matter.52) A mathematical model of FFR on the base of welding current, feeding speed and component of weld wire was established.53)
Currently most of the welding material is developed under traditional systems, which focus on the maximization of material performance rather than considering any adverse impact to the environment. Environment-performance coordinated welding consumable overall considers both performance and possible harm caused to the environment. This includes productivity and operating advantages, as well as environmental compatibility (including production process environmental compatibility and operating process compatibility). Research into welding consumable’s environmental load and the environmental impact of the welding product life cycle has just started under the development of new generation low toxicity and low fume welding consumables. The emission standard of welding will likely become stricter. Because of this, the evaluation of the environment load of the main ingredients and supplementary materials will have important significance for development of environment coordinated welding consumables. The environmental load of the core wire and four supplementary consumables including marble, mica plate, feldspar and fluorite are evaluated as shown in Table 11.54) The core wire has the largest environmental load. The factor is 68.62. The corresponding factor for marble, feldspar and fluorite during the production process is 0.39. Compared with these three materials, the factor for mica production is 2.80, larger than the total sum for the above three materials, which is related to the production process for mica. However, the environmental impact of these four materials is still less than that for the core wire. This is because their production processes and production waste are simple. A life cycle assessment (LCA) to the core wire using a fuzzy assessment method. This included steel strip and supplement production and transportation, the production of the core wire, and the impact of the welding fume on the welder was made as shown in Table 12.55) It can be seen that the production of steel strip constitutes the largest environmental load. Among the supplements, iron powder and mineral powder have little impacts on the environment. Ferrosilicon, ferromanganese and ferrotitanium have moderate impact, among which ferromanganese has the largest impact, followed by ferrosilicon, and the impact of ferrotitanium in last place.
| Mineral substance | Type | Crushing | Screening | Granding | Wet cleaning | Total influence factor |
|---|---|---|---|---|---|---|
| Marble | Dust | 1(0.14) | 1(0.12) | 1(0.13) | – | 0.39 |
| Process influence | 0.14 | 0.12 | 0.13 | – | 0.39 | |
| Feldspar | Dust | 1(0.14) | 1(0.12) | 1(0.13) | – | 0.39 |
| Process influence | 0.14 | 0.12 | 0.13 | – | 0.39 | |
| Fluorite | Dust | 1(0.14) | 1(0.12) | 1(0.13) | – | 0.39 |
| Process influence | 0.14 | 0.12 | 0.13 | – | 0.39 | |
| Mica | Dust | 4(0.24) | 4(0.12) | 4(0.13) | 4(0.21) | 2.80 |
| Process influence | 0.96 | 0.48 | 0.52 | 0.48 | 2.80 |
a Integer is influence amplitude, and value in bracket is corresponding weight coefficient. Environmental factor is influenced by influence amplitude multiplied by weight coefficient.
| Item | Steel belt | Ferrosilicon | Ferromanganese | Titanium white powder | Iron powder | Mineral powder |
|---|---|---|---|---|---|---|
| Consumption of resources | 0.96 | 0.01 | 0.01 | 0.02 | 0 | 0 |
| Energy consumption | 0.80 | 0.02 | 0.04 | 0.03 | 0.08 | 0.03 |
| Emission of three wastes | 6.143 | 0.614 | 0.106 | 0.051 | 0.025 | 0.051 |
(1) From stick electrode to solid welding wire to copper-free solid welding wire, flux-cored developed into metal-cored wire, and seamless copper FCW developed into seamless copper-free metal-cored wire. Slag and gas alloy protection have developed into reduced slag protection and increased alloy and gas production, with gas and alloy protection being especially prominent. In different regions, the protection gas can be pure CO2 or Ar+CO2 or ternary, quaternary multi-component gas. All these developments have included automation, high efficiency, environment protection, and low cost.
(2) Further research in welding metallurgy has controlled impurities such as N, H, O, S and P. The values of P, N and H can be decreased, and the appropriate value of O can be got. The weld becomes deeply purified. By reducing the diffusible hydrogen content in weld metals and combining microstructure and welding technology, crack resistance can be improved. By oxide metallurgy, the solidification processes and phase transformations of weld metals can be accurately controlled in arc welding, and finer AF can be obtained. The optimization of lower bainite (LB), lath martensite (ML) and residual austenite further toughen the weld, to match higher strength steel.
(3) Through further research on welding slag, problems such as deoxidation can be controlled, and the oxygen content in the arc weld can be reduced or controlled to meet the demand of weld oxide metallurgy. Deoxidation in the acid slag system and overcoming the problem of poor welding operability in the basic slag system, have improved the technology and cleanliness of arc welding consumables.
(4) It is a requirement of sustainable development to reduce the quantity of weld fume and its harm to welders. A new type of friendly-environment welding consumables with lower fume and spatter and better operability performance should be developed. LCA evaluation of production procedure of welding consumables should be emphasized to realize eco-material, eco-process and eco-solution gradually.
(5) Figure 15 shows trend of the ratio of sales and research expenditure and expenditure of R&D per regular researcher of general industries together with the Japanese iron and steel industry.3) The insufficiency in R&D of welding consumable can be found. So, more investment in R&D of welding consumable is expected.
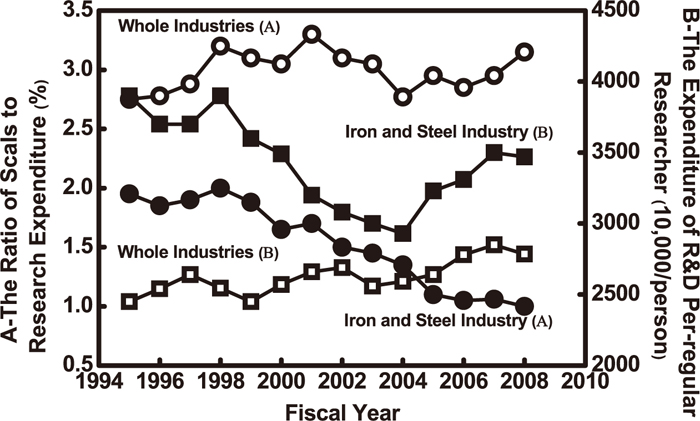
Trend of the ratio of sales and research expenditure and expenditure of R&D per regular researcher.3)