2014 Volume 54 Issue 9 Pages 2084-2088
2014 Volume 54 Issue 9 Pages 2084-2088
Thermal diffusivity and conductivity of oxide scales formed on steel plates are essential to control the cooling rate of the steel in the hot-rolling process. A flash method was applied to measure the thermal diffusivity of oxide scales. Sample specimens were commercial hot-rolled plates on which about 25 μm-thick oxide scales were formed. The results of the flash measurement with the analysis for two-layered sample provided an average thermal diffusivity value of 7.3 × 10–7 m2s–1 for the oxide scale. This value produces a thermal conductivity value of 3.8 W m–1 K–1. The future application of this measurement and analysis technique was also discussed to derive the thermal diffusivity and conductivity of oxide scales formed in actual hot-rolling processes.
Cooling rate control in the hot-rolling process is essential to obtain qualified steels.1,2,3) After being ejected from a heating furnace, a steel slab is easily oxidised and oxide scales are formed on the surface of the slab during the hot-rolling process although the oxide scale is frequently removed by descaling water. The oxide scale mainly consists of iron oxides of Fe1–xO, Fe3O4 and Fe2O3 layers,4,5,6) and plays an important role in heat transfer7,8,9,10,11) between hot steel and cooling water. The thermal conductivity of oxide scales is considered to be smaller than that of steel (c.a. 20–50 Wm–1K–1).12) This suggests that oxide scales are a resistance factor for the heat flow, leading to large temperature gradient in the thickness direction in spite of their small thicknesses. Therefore, thermal conductivity and diffusivity of oxide scales formed on steel are essential data to control the cooling rate of steel during the hot-rolling process.
To the best of the authors’ knowledge, there is, however, only one report for thermal diffusivity of an oxide scale.13) Taylor et al. measured on FeO film with the thickness of 0.35 mm grown on a 1 mm-thick iron plate. Several other reports are also available about sintered samples of FeO, Fe3O4 and Fe2O3;14,15,16,17) however, there are large discrepancies between the reported values. For example, the thermal diffusivity of FeO ranges from 4 to 7 × 10–7 m2s–1 at room temperature.14,15) The difference might come from the difference in the sample preparation processes. Slowik et al.14) measured on sintered FeO samples with 32% porosity and estimated thermal diffusivities of FeO with no porosity. Akiyama et al.15) reported thermal diffusivity and conductivity for sintered iron oxides. These suggest that it is difficult to estimate the thermal diffusivity of oxide scales on steel from the chemical compositions and temperature only using those extant data. Thus, it is of importance to conduct measurements on actual scales formed on steel.
The conventional techniques for thermal diffusivity and conductivity measurements such as the laser flash method,18,19,20) hot strip method21,22,23,24,25,26,27) etc. require bulk samples as specimen. In other words, the scale layer of 10–100 μm thick is difficult to be measured by those techniques. Another problem is that the scale layer tightly covers the steel surface, so that it is difficult to separate them. Thus, if any measurement is conducted on the oxide scale together with the steel plate, an analysis technique to separate thermophysical properties of both thin oxide layer and steel substrate must be required. Baba28) has reported measurement and analysis methods of thermal diffusivity for a multi-layered sample based on the flash technique. Akoshima et al.29) has applied this technique to obtain the thermal diffusivity of the CoNiCrAlY bond coating prepared on the Ni-based superalloy substrate by the laser flash technique. They succeeded in analysing the thermal diffusivity of the bond coating layer at room temperature. In the present study, thermal diffusivity of an oxide scale formed on hot-rolled steel is quantitatively measured at room temperature by a flash technique with the analysis for the multi-layered sample. Based on the measurement results, future issues are discussed in order to utilize our measurement procedure to improve the practical hot-rolling process.
Figure 1 shows a schematic illustration of the flash measurement applied in this study. The sample consists of two parts: an oxide scale layer and a steel substrate. The steel surface is heated by a Xenon flash light, and the temperature change at the oxide scale surface is measured with a radiation thermometer. Figure 2 shows a schematic representation of the temperature change, which is normalised by the maximum temperature rise. The hatched area in this figure is defined as the areal heat diffusion time (A).28) For the present system, the value of A can be related to thermal diffusivities of the scale and the substrate as follows,
| (1) |

Schematic illustration of flash measurement applied to “oxide scale/steel substrate” two-layered composit system.

Schematic representation of temperature change obtained by laser flash measurement and definition of areal heat diffusion time.
where D and d are the thermal diffusivity and the thickness, respectively, and the subscripts “steel” and “oxide” represent the steel substrate and the oxide scale layer, respectively. In addition, Γ = Cpρd, where Cp is the specific heat capacity and ρ is the density. The thermal diffusivity of oxide scale (Doxide) can be calculated from Eq. (1) by substituting values for A, Dsteel, Cp, ρ and d. In this study, the values of A, d and Dsteel are measured; in particular, the last is obtained by the flash method for a steel substrate using the half time analysis.18) Reported values are used for Cp, and ρ of steel and oxide scale.11,12)
Commercial heavy steel plates having 9 mm thickness with an oxide scale were used for this study. The chemical compositions of the steel are shown in Table 1. The plate was produced by the process as follows: a slab heated in an annealed furnace was hot-rolled by two rolling machines, i.e. a roughing mill and a finishing rolling mill, followed by air cooling to room temperature. During the process, the oxide scale formed on the steel plate. The steel plate was cut into a piece of 10 mm × 10 mm in size and 0.3 mm or 0.5 mm in the thickness, which was used for flash measurements as shown in Fig. 1. The thickness, structure and chemical compositions of the oxide scale were analysed by a field emission-scanning electron microscope with an energy dispersive spectrometer (FE-SEM/EDS). For this structural analysis, another sample was prepared in the same manner as that for the flash measurement.
| Fe | C | Si | Mn | P | S | N | T. Al |
|---|---|---|---|---|---|---|---|
| Bal. | 0.17 | 0.22 | 0.48 | 0.012 | 0.002 | 0.009 | 0.036 |
The samples for thermal diffusivity measurement were spray-coated with graphite particles on both surfaces and placed in the flash apparatus, which was optimised for the measurement of a square-plate sample. The steel surface was heated by a flash light with a pulse width of 0.1 or 0.2 ms from a Xenon lamp. Because of weak optical power of the flash light due to the short pulse width, the transient temperature data were averaged from six separate measurements. After these measurements, the oxide scale was removed from the steel substrate using an HCl solution. These bare steel substrates were also spray-coated with graphite particles, and the flash measurements were carried out again to obtain the thermal diffusivity of the steel substrate using the half time analysis.18) The thickness of the initial sample and the bare steel substrates was measured using a dial gauge to confirm all the oxide scale was removed. Here the thickness of the graphite coatings was negligibly small.
Figure 3 shows a backscattered electron (BE) image of cross-section of the sample. A continuous oxide scale is formed on the steel. The surface of the oxide scale is flat because the steel plate was hot-rolled during the manufacturing process. In Fig. 3 the oxide scale consists of Fe2O3, Fe3O4 and FeO layers from the surface, and there is an oxide layer containing Si between the FeO layer and the steel substrate. Good adhesion can be seen between each oxide layer and the steel substrate. The Fe2O3 layer is thin and considered to have been formed after the hot-rolling process. Accordingly, the composition of oxide scale is roughly estimated to be 30 Fe3O4-70 FeO in vol%, where the Fe2O3 layer can be ignored. The average thickness of the oxide scale is approximately 25 μm.
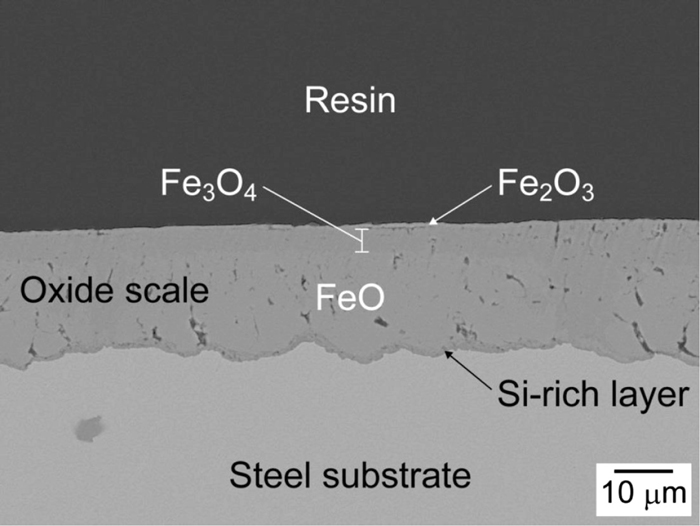
Cross-sectional BE image of oxide scale formed on heavy steel plate.
Table 2 gives the thickness of the oxide scale layer (doxide) derived from SEM images, and those for the bare steel substrates (dsteel) and the initial samples (dinitial) measured by the dial gauge. The difference between dinitial and dsteel was caused by the dissolution of the oxide scale into HCl solution. The reduced thickness is in good agreement with the thickness of oxide scale observed by SEM, suggesting that all the oxide scale layer was removed by the dissolution process.
| Sample | doxide/μm | dsteel/μm | dinitial/μm | Areal heat diffusion time, A, with oxide scale/s | Dsteel/m2s–1 | Doxide/m2s–1 |
|---|---|---|---|---|---|---|
| 0.3 mm-thick | 25a) | 343 ± 8b) | 369 ± 5b) | 1.99 × 10–3 | 1.49 × 10–5 | 6.7 × 10–7 |
| 0.5 mm-thick | 25a) | 487 ± 7b) | 509 ± 4b) | 3.35 × 10–3 | 1.51 × 10–5 | 7.8 × 10–7 |
Figure 4 shows the results of flash measurements on 0.3 mm-thick and 0.5 mm-thick samples with and without the oxide scale. The sharp pulses recorded around t = 0 are attributed to the scattered light from the Xenon flash. The samples having the oxide scale show slower temperature increase than the respective bare steel sample. This suggests that the effect of the oxide scale can be detected by this technique. The thermal diffusivity of the samples is obtained as follows. The areal heat diffusion time (A) of the samples with the oxide scale is calculated according to Fig. 2. In this calculation, the pulses around t = 0 are omitted. From the temperature change of the samples without the oxide scale, the thermal diffusivities of the steel substrates are calculated to be 1.49 × 10–5 m2s–1 and 1.51 × 10–5 m2s–1 for 0.3 mm-thick and 0.5 mm-thick samples, respectively. The obtained thermal diffusivity of the steel is close to that reported for low carbon steel,12) 1.4 × 10–5 m2s–1. Those values along with the thickness data are also listed in Table 2. To determine the thermal diffusivity of the oxide scale, the density and specific heat capacity data for the oxide scale and the steel substrate are also required according to Eq. (1) and are summarised in Table 3. The values for the oxide scale have been estimated from the composition (30 vol% Fe3O4 – 70 vol% FeO) assuming the additivity. Using the data listed in Tables 2 and 3, the thermal diffusivities of the oxide scale have been determined to be 6.7 × 10–7 m2s–1 and 7.8 × 10–7 m2s–1 for 0.3 mm-thick and 0.5 mm-thick samples, respectively, as listed in Table 2. The difference would come from the inhomogeneity of the thickness of the oxide scale. On the basis of Eq. (1), uncertainty of the thickness directly affects the value of thermal diffusivity. The thermal diffusivity of the oxide scale is determined to be 7.3 × 10–7 m2s–1 as an average.

Temperature change measured by flash method for (a) 0.3 mm-thick and (b) 0.5 mm-thick samples with and without oxide scales. (Online version in colour.)
The average thermal diffusivity value of the oxide scale determined in this study is compared with reported values for iron oxides in Fig. 5 just for reference. Taylor et al.13) have measured the thermal diffusivity of an FeO film 0.35 mm thick grown on a 1 mm-thick iron plate at temperatures from 623 to 753 K. The sample structure was similar to that in this study. Other data are for sintered iron oxides. The thermal diffusivity of the oxide scale measured in this study exists near a line extrapolated from the data reported by Taylor et al.13) This would be because the main component of the oxide scale is FeO. This figure also suggests that it is not possible to estimate the thermal diffusivity of the scale from the chemical composition with previously reported thermal diffusivities for sintered iron oxides even at room temperature.

The thermal conductivity of oxide scale has been calculated as 3.8 Wm–1K–1 from the obtained thermal diffusivity value according to the following equation,
The following discusses application of the flash method with the analysis for two-layered sample to determination of thermal diffusivity and conductivity of oxide scales formed in hot-rolling process from the viewpoints of (i) measurement and analysis technique and (ii) conditions in the hot-rolling process.
(i) Measurement and Analysis Technique
Akoshima et al.19) has applied the laser flash method to two- or three- layered samples at elevated temperatures. Firstly, they used samples of CoNiCrAlY bond coating prepared on a Ni-based superalloy substrate. In this case, it was difficult to determine the thermal diffusivity for the bond coating layer because both the bond coating layer and the substrate are metals and have the same magnitude of thermal diffusivities. On the other hand, on the measurements for the two-layered samples, coated with yttria stabilized zirconia (YSZ), they found that the flash signal showed the influence of the top YSZ coating layer since YSZ has much smaller thermal diffusivity than the bond coating and the substrate. In this study the measured thermal diffusivity of the scale is about one order of magnitude smaller than that for the steel substrate and this relation would not change at higher temperatures. This suggests that different flash signals might be obtained for a steel substrate with/without an oxide scale layer as shown in Fig. 4 even at elevated temperatures, and the thermal diffusivity of the oxide scale and steel substrate can be determined.
In the analysis to derive the thermal diffusivity of the oxide scale, the effect of the thermal resistance between the oxide layers has been considered to be negligibly small; for example, the thermal resistance of grain boundary has been reported for alumina ceramics to be approximately 10–8 m2KW–1,30) which value is sufficiently small compared to a thermal resistance value for the oxide scale, 10–6 m2KW–1, which has been calculated from the thermal conductivity and the thickness.
(ii) Conditions in Hot-rolling Process
The steel surface is covered with water or its vapor in the actual hot-rolling process. This suggests that the water penetrates into the oxide scale, which may cause the change of the thermal properties of oxide scales. The general flash measurement is, however, conducted in vacuum since the analytical solution for flash measurements has been given under adiabatic condition. The effect of atmosphere gas in a flash measurement results in heat loss, which can be observed in the measured temperature change. The thermal diffusivity determination in non-adiabatic condition has been discussed,19,31,32) which would be applied to future measurements. Furthermore, along with the thermal diffusivity and conductivity measurements of oxide scales under the simulated conditions of the practical process, the structure of oxide scales also have to be observed, since database of the thermal diffusivity and conductivity of oxide scales including the structural model will enable the thermal properties to be predicted in the future.
Thermal diffusivity was measured for oxide scales formed on commercial hot-rolled plates using the flash method with the analysis for two-layered sample at room temperature. The measured thermal diffusivity is 7.3 × 10–7 m2s–1 as an average, which produces a thermal conductivity value of 3.8 W m–1 K–1. The thermal diffusivity of the oxide layer is about one order of magnitude smaller than that for the steel substrate. This suggests that there is a possibility of measuring thermal diffusivity and conductivity of oxide scales formed on steel substrate by the flash method with the analysis for two-layered sample.
This work was supported by the research group for “Thermophysical Properties of Oxide Scale” in Rolling Theory Committee, The Iron and Steel Institute of Japan. The authors are grateful for the support and useful advices from the committee members.