2014 Volume 54 Issue 9 Pages 2120-2124
2014 Volume 54 Issue 9 Pages 2120-2124
The molar volumes of binary M–Si (M=Fe, Ni, and Ge) alloys in a liquid state were obtained from their densities measured using electromagnetic levitation coupled with a static magnetic field. We have systematically analyzed the molar volume as a function of composition. Although the concentration dependence of the molar volume of the liquid Ge–Si alloys obeys a linear law which is indicative of an ideal mixture, in comparison, that of the Ni–Si alloys shows a large discrepancy from the linear law as well as the Fe–Si system. The interaction between the constituent atoms was discussed with a view to analyzing the partial molar volume.
The study of binary silicides has been extensive over a long period because of their wide variety of applications as industrial materials. In particular the Fe–Si system has several compounds such as Fe3Si for a ferromagnetic material,1) FeSi for a magnetic semiconductor stable at high-temperature,2) and FeSi2 and Fe2Si5 for thermoelectric materials.3) The Ni–Si system forms compounds at similar compositions as the Fe–Si system and their electronic and magnetic properties have also received widespread interest.4,5) Although Ge–Si alloys show no formation of compounds, their electronic properties have been paid attention due to their remarkable potential for application.6,7) Hence, a large number of studies have been reported for their properties in thin films, in addition, there are several reports for single crystals grown by the Czochralski technique.8,9)
It is widely known that reliable data of thermophysical properties such as thermal conductivity, density, surface tension, and viscosity are indispensable to control a heat and mass transfer process at the solid-liquid interface during crystal growth. In addition, the thermophysical properties of the liquid phase are of particular interest since they play an important role in defining the thermodynamic state and in understanding relations with microscopic structures of the melt. Therefore, a comparative study of these Si alloys is desirable in order to clarify the relationship between the compound forming compositions and the concentration dependence of thermophysical properties especially the density of the melts, since the density is the most fundamental quantity to understand physicochemical properties. However, experimental measurements of the thermophysical properties of high-temperature melts are difficult due to contamination, mainly from contact materials of the sample container. In recent decades, various containerless techniques have been developed to study physical properties in the liquid state of materials with high melting temperatures (in excess of 2000 K).10,11) Fukuyama et al.12) developed an experimental technique using electromagnetic levitation (EML) coupled with a static magnetic field for non-contact measurement of thermophysical properties of high-temperature melts. Since a static magnetic field of the order of several tesla can suppress surface oscillations and translational motions of a liquid droplet levitated electromagnetically,13) this technique enables us to obtain accurate values of thermophysical properties such as thermal conductivity, heat capacity,14,15,16,17) spectral emissivity,16,17) surface tension,18) and density.19,20,21)
Several reports have been published on the liquid density of the above-mentioned silicon alloys,19,20,21,22,23,24) yet the range of composition covered is still inadequate to perform a systematic study and there is some remaining uncertainty concerning the absolute value and its temperature dependence. In addition, precise density values are essential to analyze structural data of liquid M–Si (M=Fe, Ni, and Ge) alloys, obtained from synchrotron x-ray diffraction experiments conducted by the present authors.25) Therefore, following the previous work,20,21) we have measured the liquid density of M–Si (M=Fe, Ni, and Ge) alloys with the EML technique coupled with a static magnetic field. The aim of this paper is to report results of systematic analysis concerning the concentration dependence of the molar volume obtained from the measured density, in order to study the mixing effect on the molar volume over an entire concentration range and wide temperature range including an undercooled liquid state.
Since the principle for density measurement using EML coupled with a static magnetic field is similar to those previously reported,19,20,21) a brief description is given below for the experimental equipment. In Fig. 1 the experimental setup for density measurements is shown. The levitation coil in a high-vacuum chamber was located at the center of the magnetic field generated using a superconducting magnet (JMTD-10T120SSFX, Japan Superconductor Technology, Inc.) with a bore diameter of 120 mm. The radio frequency generator (maximum power of 10 kW, EASYHEAT, Ameritherm, Inc.) operates at a frequency from 150 to 400 kHz, which depends on electrical properties due to the sample environment. Due to the low electrical conductivity of Si-based alloys as well as Ge and Si, the eddy current induced from the RF coil is inadequate to levitate and heat the sample when compared with typical metallic samples. Therefore, a high-power semiconductor laser (maximum power of 140 W, wavelength of 807 nm, NBT-S140-mk II, JENOPTIK Laserdiode Japan Co., Ltd.) was installed to heat and melt the sample before levitation.

Schematic illustration of the experimental system developed for the measurement of thermophysical properties with electromagnetic levitation coupled with a static magnetic field generated by a superconducting magnet.
The temperature of the levitated droplet was obtained by calibrating the output signal of the single color pyrometer using Planck’s law, at the liquidus temperature of the alloy.15)
The density of the levitated droplet was determined using an image processing method.26) To image the sharp edge of the droplet accurately, we adopted a shadowgraph technique which eliminates intense radiance from the high-temperature droplet. A laser light source with a wavelength of 532 nm was mounted through a beam shaper for back illumination. In addition, a high-speed video camera (FASTCAM-512PCI, Photron, Ltd.) was used to obtain shadow images of the droplet from the side. The high-speed video camera was operated at a frame rate of 500 Hz and images at a resolution of 512 × 512 pixels were recorded continuously during the cooling process of the sample.
2.2. Estimation of DensityThe volume of the levitated droplet can be determined by an image sectioning method of the droplet profile. The radius, r, from the center of mass of the droplet was fitted with a series of Legendre polynomials, Pn, as a function of the polar angle, θ, as
| (1) |
The volume, V, of the droplet is then estimated by assuming that the shape of the droplet is symmetrical around the vertical axis, that is,
| (2) |
In this procedure, the conversion of units from pixel to length was made using the image of a calibration sphere. The mass of the sample was measured before and after levitation to account for any volume change due to evaporation of the sample. The density, ρ, can then be estimated from the mass and volume.
Binary alloys of a M–Si (M=Fe, Ni, and Ge) system were prepared from constituent elements by means of conventional arc melting in a highly purified Ar gas atmosphere. The compositions of the alloy were chosen as close to the compound forming and the congruent compositions in the case of the Fe–Si and Ni–Si systems.
In Figs. 2(a)–2(c) the concentration dependence of the density of the liquid Si alloys as a function of temperature is shown. The temperature range covered not only the liquid phase beyond the liquidus temperature, but also the undercooled liquid state. The densities of the Ge–Si system show widely scattered values in particular for the low temperature and high concentration ranges of Ge, when compared with the other two systems. This is due to a deformation of the sample droplet during a cooling process by blowing He gas to achieve the low temperature condition for the Ge-rich Si alloys which have lower liquidus temperature than that of the other two alloy systems. In the case of the Fe–Si alloys, the values of the density are in good agreement with the reported values obtained from the x-ray sessile drop technique22) (not shown). For the temperature range investigated in the present study, the temperature dependence of the density can be fitted by a linear function as
| (3) |

Temperature dependence of the density of the Fe–Si (a), Ni–Si (b), and Ge–Si (c) liquid alloys. The values of chemical composition are expressed in terms of atomic percent of Si content.
| System | Concentration | Liquidus temperature | Density at TL | Temperature coefficient | Expanded uncertainty with coverage factor of 2 |
|---|---|---|---|---|---|
| at. % Si | TL/K | ρL / kg m–3 | α/kg m–3K–1 | U / kg m–3 | |
| Fe–Si | 0 | 1808 | 7162 | –0.735 | 93 |
| 5 | 1793 | 6705 | –1.059 | 89 | |
| 10 | 1745 | 6539 | –0.919 | 85 | |
| 15 | 1681 | 6345 | –0.881 | 81 | |
| 20 | 1595 | 6380 | –0.708 | 82 | |
| 25 | 1553 | 6065 | –1.065 | 75 | |
| 34 | 1473 | 5827 | –0.731 | 73 | |
| 38 | 1561 | 5644 | –0.724 | 70 | |
| 40 | 1603 | 5675 | –0.615 | 73 | |
| 45 | 1655 | 5278 | –0.755 | 67 | |
| 50 | 1693 | 5051 | –0.773 | 64 | |
| 55 | 1657 | 4773 | –0.716 | 60 | |
| 60 | 1603 | 4572 | –0.581 | 58 | |
| 70 | 1520 | 4022 | –0.671 | 49 | |
| 75 | 1486 | 3654 | –0.609 | 44 | |
| 80 | 1583 | 3519 | –0.386 | 45 | |
| 90 | 1643 | 2949 | –0.260 | 39 | |
| 100 | 1687 | 2580 | –0.184 | 36 | |
| Ni–Si | 0 | 1728 | 7783 | –0.671 | 96 |
| 10 | 1623 | 7523 | –0.773 | 91 | |
| 20 | 1451 | 7015 | –0.694 | 82 | |
| 26 | 1503 | 6859 | –0.780 | 81 | |
| 30 | 1490 | 6713 | –0.637 | 79 | |
| 33 | 1579 | 6530 | –0.650 | 78 | |
| 35 | 1567 | 6406 | –0.640 | 76 | |
| 40 | 1451 | 6137 | –0.558 | 72 | |
| 45 | 1278 | 5921 | –0.527 | 68 | |
| 50 | 1265 | 5480 | –0.482 | 62 | |
| 67 | 1389 | 4264 | –0.319 | 48 | |
| 67 | 1389 | 4251 | –0.280 | 48 | |
| 70 | 1434 | 4084 | –0.262 | 47 | |
| 80 | 1551 | 3549 | –0.256 | – | |
| 90 | 1632 | 3019 | –0.211 | – | |
| Ge–Si | 0 | 1211 | 5616 | –0.508 | 71 |
| 13 | 1353 | 5241 | –0.434 | 66 | |
| 25 | 1433 | 4894 | –0.457 | 61 | |
| 38 | 1493 | 4536 | –0.367 | 57 | |
| 50 | 1543 | 4147 | –0.325 | 53 | |
| 63 | 1583 | 3835 | –0.223 | 50 | |
| 75 | 1623 | 3400 | –0.246 | 45 | |
| 88 | 1653 | 2994 | –0.199 | 41 |
In thermodynamics, density is an intensive quantity, whereas volume is an extensive quantity. Hence, we have analyzed the molar volume to discuss effects on mixing Si and another element in the liquid state. In Fig. 3 the molar volumes of the liquid Si alloys are shown, which were estimated from the measured density at the isothermal condition. The molar volume of the Ge–Si alloy decreases monotonically with an increase in Si content and smoothly obeys the linear law. In contrast, the molar volume of the Fe–Si alloy increases with increasing Si concentration as well as that of the Ni–Si alloy. In addition, large discrepancies from the linear law can be observed in these systems when compared with the Ge–Si system.
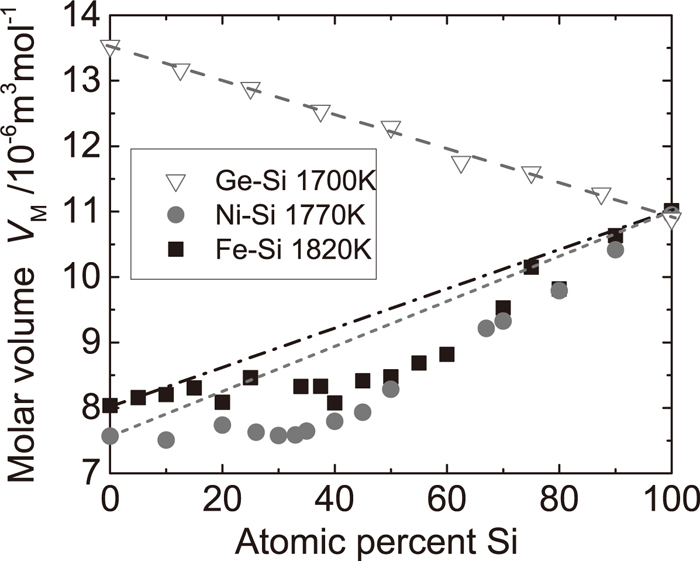
Molar volume of liquid Si alloys as a function of composition. Dotted lines indicate the estimated molar volumes assuming the concentration dependence obeying the linear law.
To clarify these differences between the systems, the excess molar volume, VE, was estimated as
| (4) |

Excess molar volume of liquid Si alloys as a function of composition.
On the other hand, the excess molar volume of the Fe–Si alloys shows large negative deviation from the linear law and the minimum near the equiatomic composition. In addition, the Ni–Si system also shows negative deviation from the linear law and a minimum around 40 at. % of Si. According to the binary phase diagram of the Fe–Si and Ni–Si systems, the composition showing the minimum in the excess molar volume corresponds to the composition that forms the congruent compounds with the highest liquidus temperature, 50 at.% of Si in the Fe–Si system and 33 at.% of Si in the Ni–Si system.28) Therefore, these results signify that the interaction between Si and Fe or Ni atoms is relatively attractive at the compound forming composition.
To obtain more insight into the mixing effect on the volume, we have estimated the partial molar volume of species i, Vi*, defined as
| (5) |

Concentration dependence of the partial molar volume of the Fe–Si (a), Ni–Si (b), and Ge–Si (c) liquid alloys.
The densities or molar volumes were determined for liquid M–Si (M=Fe, Ni, and Ge) alloys in a wide temperature range over the whole concentration range of the alloys. The density showed linear temperature dependence for all compositions of alloys investigated in this study. The molar volume estimated as a function of composition at constant temperatures was analyzed systematically. It was found that the Ge–Si alloy shows ideal mixing behavior with a nearly vanishing excess molar volume. On the other hand, in the case of the Fe–Si and Ni–Si liquid alloys, negative values of the excess volume and a drastic change of the partial molar volume were observed around the compound-forming composition in the solid state. This indicates that the interatomic interaction in transition metal-Si alloys increases drastically with Si content.
The authors would like to thank Professor T. Hibiya (Keio University) and Professor S. Ozawa (Tokyo Metropolitan University) for their helpful discussions. This work was supported financially by SENTAN, Japan Science and Technology Agency (JST).