2015 Volume 55 Issue 8 Pages 1699-1701
2015 Volume 55 Issue 8 Pages 1699-1701
A new catalytic combustion-type CO gas sensor was devised by using the precious metal-free CO oxidizing catalyst of 15.9 wt% La0.87Co1.13O3-loaded Ce0.67Zr0.18Sn0.15O2.0. Since the 15.9 wt% La0.87Co1.13O3-loaded Ce0.67Zr0.18Sn0.15O2.0 catalyst oxidizes CO completely at 130°C, the sensor showed smooth and reproducible response to CO gas at ca. 130°C. Moreover, the sensor exhibited a linear relationship between response and CO gas concentration, along with short 50% response time of 20–40 s.
Carbon monoxide (CO) is a highly toxic gas which causes serious health damage even at low concentrations when it is inhaled. Since CO gas cannot be detected by our senses because of its colorless and odorless nature, detection equipment is always necessary to prevent accidents caused by the exposure to CO. Therefore, it is desired to install compact and inexpensive CO sensor at every site where CO gas may be emitted.
Until now, the various types of compact CO gas sensors have been developed based on semiconductor,1,2) potentiostat,3) and catalytic combustion-type.4,5) Although semiconductor-type CO gas sensors have merits of stable sensor performance with low cost, this type of sensor has a basic problem in gas selectivity because other coexisting gases also adsorb on the semiconductor surface which produces a signal as well as CO. Potentiostat-type CO gas sensor is nowadays widely used owing to its selective CO detection, but they cannot operate for a long period because of the eventual evaporation of liquid electrolyte in the device. In contrast, catalytic combustion-type CO gas sensor can show the stable sensing performance for a long period because of its simple detection system which is combined by a Pt coil and a CO oxidation catalyst. The detection mechanism is as follows. When CO gas is oxidized on the catalyst, combustion heat related to the amount of CO generates. Then, the temperature of the Pt coil coated with the catalyst rises by the generated combustion heat, causing the electrical resistance increase of the Pt coil. Since the resistance change should be approximately proportional to the temperature change, the sensor signal (resistance change) is also exactly proportional to the amount of CO gas oxidized by the catalyst, in other words, CO gas content in the measuring atmosphere. However, conventional catalytic combustion-type sensors have a problem in selectivity. Because the traditional catalysts applied (Pt/Al2O3 or Pd/Al2O3) can work at several hundred Celsius degrees at which other gases such as volatile organic compounds (VOCs) also burn out, leading to certain responses to other gases. For overcoming this problem, it is essential to develop the novel catalyst which can oxidize only CO at lower temperatures at which gases other than CO are not oxidized.
Recently, we have fabricated a low-temperature operative catalytic combustion-type CO gas sensor6) by employing 10 wt% Pt/Ce0.68Zr0.17Sn0.15O2.0 as the CO oxidizing catalyst which was also developed by us. Since this catalyst oxidized CO completely at 65°C, quantitative CO detection was successfully achieved at such a low temperature of 70°C with the 50% response time (the time required for the electrical resistance of the device to reach 50% of the steady value eventually obtained at a given CO gas level) of 130 s. Moreover, we have succeeded in improving the response time (50% response time: 20–30 s) of the sensor by adding the high thermal conductive material of aluminum nitride (AlN) as an intermediate heat transfer layer between the Pt coil and the catalyst.7) Although we have succeeded in developing the low temperature operative catalytic combustion-type CO gas sensor, the 10 wt% Pt/Ce0.68Zr0.17Sn0.15O2.0 catalyst contains precious metal of Pt whose consumed amount should be saved. In the light of practical use, especially in cost, it is desired to use the Pt-free catalyst.
From such a demand, in this study, we focused on LaCoO3 which has been reported to show catalytic activity toward CO oxidation and whose CO oxidation activity was improved by loading on the material with high surface area,8) and we developed a Pt-free CO oxidizing catalyst of LaCoO3-loaded Ce0.67Zr0.18Sn0.15O2.0. The LaCoO3-loaded Ce0.67Zr0.18Sn0.15O2.0 catalyst is expected to oxidize CO at low temperatures compared with other precious metal-free catalyst9,10,11) because the Ce0.67Zr0.18Sn0.15O2.0 support has not only high surface area but also to show the co-catalytic effect by the lattice oxygen migration similar to the 10 wt% Pt/Ce0.68Zr0.17Sn0.15O2.0 catalyst. In this paper, we devised a catalytic combustion-type CO gas sensor using the LaCoO3-loaded Ce0.67Zr0.18Sn0.15O2.0 catalyst and investigated its sensing performance.
The Ce0.68Zr0.17Sn0.15O2.0 solid solution was synthesized by the sol-gel method, as described in our previous paper.6) Then LaCoO3 was loaded onto the Ce0.68Zr0.17Sn0.15O2.0 support to yield 15.9 wt% La0.87Co1.13O3-loaded Ce0.67Zr0.18Sn0.15O2.0 catalyst by the following procedure: 3.0 mL of 0.1 mol L−1 La(NO3)3 solution and 3.0 mL of 0.1 mol L−1 Co(NO3)2 solution were added to the 0.36 g of Ce0.68Zr0.17Sn0.15O2.0 solid. Then 1.26 mL of 1.0 mol L−1 cytric acid solution and about 10 mL of deionized water were added and the mixed solution was stirred for 5 h at room temperature. After then, the solvent was removed off at 80°C by using hot stirrer and the obtained powder was heated at 140°C for 1 h. The obtained powder was calcined at 700°C for 6 h in air.
The sample was identified by X-ray fluorescence (Rigaku, ZSX100e) and X-ray powder diffraction (XRD) (Rigaku, SmartLab) analyses with the Cu-Kα line. CO oxidation activity of the 15.9 wt% La0.87Co1.13O3-loaded Ce0.67Zr0.18Sn0.15O2.0 catalyst was investigated by a conventional fixed-bed flow reactor by flowing 1000 ppm CO diluted with air at the space velocity of 20000 L kg−1 h−1.
The sensor element was fabricated using a coil formed from 30 μm diameter of Pt wire. Aluminum nitride (Toyo Aluminum K. K.) was dispersed in ethylene glycol to form a slurry and then, the slurry was loaded onto the Pt coil. After then, the coil was heated at approximately 300°C by applying a dc voltage of 2 V to evaporate the ethylene glycol. The 15.9 wt% La0.87Co1.13O3-loaded Ce0.67Zr0.18Sn0.15O2.0 catalyst was dispersed in ethylene glycol and was coated over the AlN layer on the Pt coil and the ethylene glycol was removed off by the same way described above. For an effective assessment of the AlN as an intermediate heat transfer layer, the volume ratio of AlN to catalyst was almost identical to that of the previous sensor employing 10 wt% Pt/Ce0.68Zr0.17Sn0.15O2.0.7) The CO sensing performance of the sensor was investigated at 132°C by using an electrometer (Advantest, R8240) in the atmosphere whose CO gas concentration was varied from 0 to 500 ppm that obtained by diluting 1000 ppm CO (Ar balance) gas with synthetic air. Regardless of the CO gas concentration, the total gas flow rate passing over the sensor was kept constant at 40 mL min−1. The sensor signal in response to exposure to CO gas was defined as (Rgas−Rair)/Rair, where Rgas and Rair are the electrical resistances of the sensor in the test gas containing CO and in pure air, respectively. The sensor response time was defined as the time required for the electrical resistance of the device to reach 50% or 90% of the steady value eventually obtained at a given CO gas level.
We have confirmed that the cationic ratio (La : Co : Ce : Zr : Sn) in the prepared solid was almost identical to the mixing one of the reactants by X-ray fluorescence analysis. From the XRD measurement of the 15.9 wt% La0.87Co1.13O3-loaded Ce0.67Zr0.18Sn0.15O2.0 (The XRD pattern is shown in Fig. 1.), only peaks assigned to the cubic fluorite-type oxide and perobskite-type oxide without any crystalline impurities were clearly observed. Furthermore, the diffraction peak angles assigned to the cubic fluorite-type oxide were shifted slightly to higher angles as compared to those of Ce0.8Zr0.2O2.0 (PDF #01-078-0693). These results clealy suggest that the celium (ionic radius of Ce3+: 0.114 nm, Ce4+ : 0.097 nm12)) ion sites in the Ce0.8Zr0.2O2.0 were partially substituted with smaller tin ions (ionic radius of Sn2+: 0.093 nm, Sn4+ : 0.071 nm13)) to form a solid solution, and the 15.9 wt% La0.87Co1.13O3-loaded Ce0.67Zr0.18Sn0.15O2.0 solid was successfully obtained.

XRD pattern for the 15.9 wt% La0.87Co1.13O3-loaded Ce0.67Zr0.18Sn0.25O2.0 catalyst.
Figure 2 shows the CO oxidation activity of the 15.9 wt% La0.87Co1.13O3-loaded Ce0.67Zr0.18Sn0.15O2.0 catalyst. The present catalyst starts to oxidize CO at 30°C and complete oxidation is realized at 130°C. Such high CO oxidation activity is considered to be realized by the co-catalytic effect of the Ce0.67Zr0.18Sn0.25O2.0 support. Lattice oxygen in the support may migrate easily to the surface through the lattice oxygen vacancies generated by the existence of a certain amount of Sn2+ ion.14) As a result, oxygen migrated to the surface also work for the CO oxidation. From this result, we can expect that the sensor detect CO gas concentration quantitatively above 130°C even in the absence of precious metal.
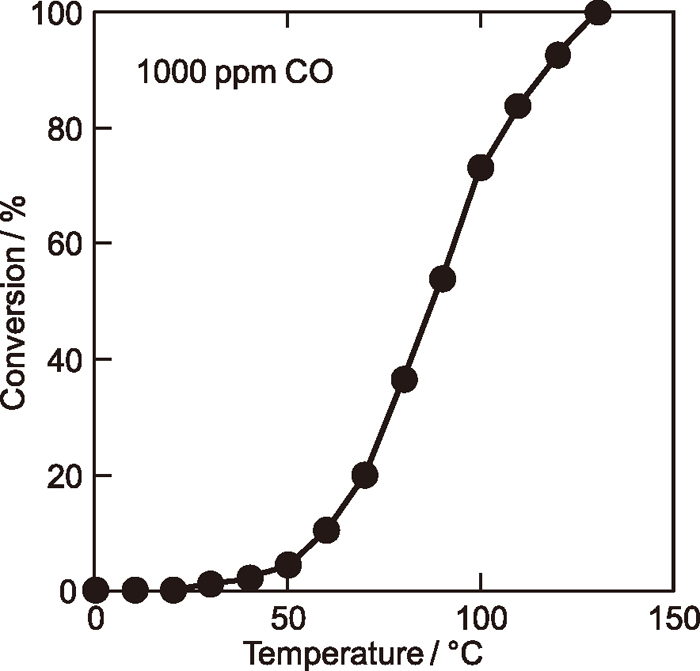
Temperature dependence of the CO conversion to CO2 for the 15.9 wt% La0.87Co1.13O3-loaded Ce0.67Zr0.18Sn0.25O2.0 catalyst.
Figure 3 displays a representative response curve for the present sensor with the 15.9 wt% La0.87Co1.13O3-loaded Ce0.67Zr0.18Sn0.15O2.0 catalyst and the AlN intermediate heat transfer layer obtained when the CO gas concentration was varied from 0 to 500 ppm and vice versa in a step-by-step manner at 132°C. The sensor signal ((Rgas−Rair)/Rair) changed smoothly and reproducibly with varying the CO gas concentration. Furthermore, the 50% and the 90% response time were 20–40 s and 50–100 s, respectively, which are similar to those (20–30 s and 90 s) of our previously reported sensor employing 10 wt% precious Pt/Ce0.68Zr0.17Sn0.15O2.0 and AlN intermediate layer.7)

Representative sensor response curve obtained by varying CO concentration from 0 to 500 ppm and vice versa at 132°C.
Figure 4 depicts the steady-state sensor signals at various CO gas concentrations at 132°C. Similar to the previous sensor reported by us,7) the present sensor produces a signal which varies in a linear fashion with changing the CO gas concentration, suggesting that the present sensor can detect CO concentration quantitatively. Although the operation temperature of the present sensor is higher than that of the previous sensor with Pt loaded catalyst,7) it is quite lower than those (250–400°C) of conventional sensors with precious metal-loading Pt/Al2O3 or Pd/Al2O3 catalyst. Moreover, the present sensor has a merit of cost performance because the present sensor employed precious-metal free catalyst of 15.9 wt% La0.87Co1.13O3-loaded Ce0.67Zr0.18Sn0.15O2.0 having the similar quick response to our previous sensor with Pt-loaded catalyst.7) From these advantages, the present sensor is considerd to be another candidate for the practical CO detecting tool.

Sensor signals at various CO concentrations for the present sensor incorporating the 15.9 wt% La0.87Co1.13O3-loaded Ce0.67Zr0.18Sn0.25O2.0 catalyst and AlN intermediate heat transfer layer at 132°C.
Present work was partially supported by the working group of WSN (Wireless Sensor Network 2012–2015) supported by The Iron and Steel Institute of Japan and by a Grant-in-Aid for Science Research (No. 24655193) from the Japan Society for the Promotion of Science.