2015 Volume 55 Issue 9 Pages 1828-1833
2015 Volume 55 Issue 9 Pages 1828-1833
Krupp–Renn process is an energy-saving technology for ferronickel production from saprolitic laterite ores, in which a semi-fused zone (1300–1350°C) in the rotary kiln is required for the coalescence of ferronickel particles. Fluxes are crucial for the liquid phase formation during the coalescence stage. In this study, effect of quaternary basicity ((CaO+MgO)/(Al2O3+SiO2) mass ratio) on the melting characteristics of laterite ore blended with various amount of CaO in 100% CO atmosphere are investigated, and the growth of ferronickel particles is also observed. It is shown that the characteristic fusion temperatures first decline and then increase with increasing of basicity, which is closely related with the presenting silicate minerals in the reduced products involving olivine, diopside, monticellite, akermanite and merwinite. At the basicity of 0.8–1.2, the characteristic fusion temperatures drop subsequently before reaching a minimum (~1300°C), due to the generation of low-melting-point diopside. The growth of ferronickel particles is improved by the presence of liquid phase.
Crude ferronickel can be produced from laterite ores by a number of pyrometallurgical processes.1,2,3,4) It is an inexpensive alternative to electrolytic nickel used for stainless steels manufacturing.5,6) Current commercial pyrometallurgical processes include the blast furnace smelting process (BF),5,6) rotary kiln-electric furnace smelting process (RKEF),7,8) and Krupp–Renn process.9)
In recent years, BF process has been going through a rapid development in China as a result of the shortage of nickel supply. The BF smelting process benefits from mature technology and existing manufacturing facilities from iron and steel industry, to produce low–nickel pig iron (2–4 wt.% Ni). This process suffers low productivity and high coke consumption in the sintering stage,10,11,12) along with the difficulty in separating slag and hot metal.13,14)
RKEF process has been widely used for ferronickel (20–40 wt.% Ni) production from saprolitic laterite ores. This process is characterized by high energy consumption. It was reported that the power consumption in electric furnace smelting stage accounts for about 50% of total operation cost.5) Additionally, it is inappropriate for processing laterite ores with high iron content and low nickel content.
Krupp-Renn process is an economic process which is initially used to reduce and smelt low-grade iron ores in rotary kiln instead of BF. The Nippon Yakin plant in Oheyama, produces ferronickel luppen by modifying the Krupp-Renn process.9,15) Oheyama process bears advantages of low energy consumption and high quality of ferronickel alloy produced.3,15,16,17) In the Oheyama process, the aggregation and growth of ferronickel particles and the separation of ferronickel from slag are crucial. In fact, large amounts of refractory silicate minerals with high melting points are generated during reductive roasting of laterite ores. Because mass transfer and ferronickel particles aggregation proceed slowly at the low temperatures, high temperatures (1300–1350°C) are required for generation of semi-fused laterite lumps, to facilitate the growth of ferronickel particles.18,19,20) This is why the process usually faced with serious issues of kiln-ringing.18,20)
Limestone and quick lime are the common fluxes used for pyrometallurgical treatment of laterite ores. Calcium oxide is able to facilitate the destruction of silicate networks, improving the reduction of nickel and iron.21) Besides, the addition of an appropriate amount of CaO facilitates the mass transfer.22) Previous studies20,23,24) on the effects of limestone on softening and melting behavior of laterite ores in reductive roasting with anthracite showed that CaO enhances liquid formation and reduces melting temperature. However, a quantitative evaluation of relationship between CaO dosage and melting behavior in the reduction process is still lacking. In this study, CaO is used to adjust the basicity of saprolitic laterite ore in the simulative experiments of Krupp–Renn process. The characteristic fusion temperatures of laterite ore with various basicities during reductive roasting in 100% CO atmosphere are determined. Additionally, the phase transformation, melting behavior and ferronickel particles growth are investigated.
The saprolitic laterite ore was taken from Sulawesi Island, Indonesia. The sample was dried at 110°C for 2 h, and then ground to 100% of the particles passing through a 0.1 mm pore size sieve. The chemical composition is shown in Table 1, the sample is characterized by high magnesia and silica contents. The contents of total iron and nickel were 22.1% and 1.91%, respectively. According to the XRD results, the laterite mainly consists of lizardite ((Mg,Fe)3Si2O5(OH)4), goethite and hematite.
| Composition | Fetotal | Ni | SiO2 | MgO | Al2O3 | CaO | Cr2O3 | LOI* |
|---|---|---|---|---|---|---|---|---|
| Content | 22.1 | 1.91 | 26.49 | 13.42 | 4.25 | 2.04 | 1.68 | 13.18 |
CaO of analytic grade was used as flux to adjust the basicity of laterite ore, and the particle size was of 100 wt.% passing a 74 μm-pore size sieve.
2.1.3. GasCO (99.9% purity) and N2 (99.99% purity) were used as the reducing and cooling agents, respectively.
2.2. Methods 2.2.1. Determination of Characteristic Fusion TemperaturesThe dried laterite ore was mixed with a certain quantity of CaO, and then the mixture was shaped by a mold into pyramids (1.5 g, bottom side length of 5 mm and vertical height of 12 mm). The schematic diagram of the controlled atmosphere furnace is shown in Fig. 1. CO gas was replenished to the horizontal alumina tube (diameter of 40 mm and length of 550 mm) and the inlet gas flow was fixed at 350 L/h. The pyramid was placed on a corundum plate and heated in the furnace with a heating rate of 10°C/min in 100% CO atmosphere from 900°C to 1500°C. The external shape change of the sample was continuously recorded by a camera every 10 s during the experiment. Three characteristic temperatures were identified to determine the soft and fusion characteristics of laterite ore and CaO mixture with various basicities. The characteristic temperatures were identified according to: (1) deformation temperature (TD), determined by the temperature at which the tip of the pyramid becomes spherical or curved; (2) sphere temperature (TS), determined by the temperature at which the whole pyramid deforms to be hemispheric; (3) flow temperature (TF): determined by the temperature at which the pyramid melts until the vertical height is less than 1.5 mm.
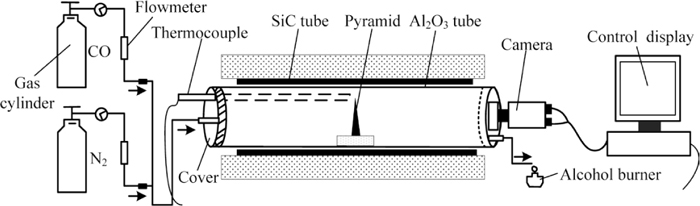
Schematic diagram of the horizontal tube furnace.
The mixture of laterite and CaO was briquetted into cylindrical briquettes (4.0 g, diameter of 10 mm and height of 15 mm) at a pressure of 5 MPa. These briquettes were dried at 110°C for 4 h subsequently. The dried briquettes were loaded into a corundum crucible and roasted in 100% CO atmosphere for 1 h with the roasting temperature varying from 1100°C to 1300°C. In all cases, the gas flow rate was 350 L/h. After roasting, the furnace was shut down and the gas was shifted from CO to N2. The crucible was moved to the low-temperature zone of the tube, and the system was cooled to room temperature with a cooling speed of 40 K/min. A part of reduced briquettes was prepared for XRD analysis, and another part of the product was fixed into an epoxy resin mould, ground and polished to prepare cross-sections for microstructure observation.
2.2.3. Measurement of Ferronickel Particles SizeThe average size of ferronickel particles was calculated by using an optical microscopy (LEICA MDI5000 M, Germany) and the software Image-Pro Plus 6.0. Firstly, as shown in Fig. 2, 16 zones were divided evenly in both edge and middle (1/2 radium) of the cross-section of reduced briquettes, and then 2 photos (200×150 μm) in each zone (32 photos in total for each specimen) were taken and saved in the computer. And then, the quantity and area of ferronickel particles in the photos were detected by using the software Image-Pro Plus 6.0. Assume particles are sphere in the three-dimensional space, indicating that these could be regarded as round in the two-dimensional space. Based on the assumption, the equivalent diameter and volume of each particle can further be calculated. Provided that the area of the edge zone (S2) is 2-time larger than that of the middle zone (S1), as the area of photo taking zone is extremely small compared to that of briquette cross-section, likewise the volume ratio. Therefore, the total volume of ferronickel particles can be calculated:
| (1) |
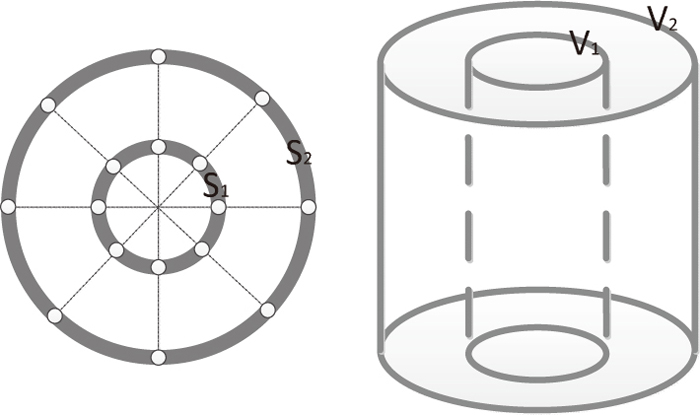
Schematic diagram for photo taking zones (circles in white) of the cross-section of reduced briquettes.
Subsequently, the relationship between cumulative volume ratios vs. diameter of ferronickel particle was plotted, and the diameter corresponding to the cumulative volume ratio of 50% was defined as the apparent mean particle size (L) of ferronickel. And the true particle size (D) was then corrected by Eq. (2).25,26)
| (2) |
In this study, X-ray diffraction patterns for the reduced briquettes were obtained by using a conventional X-ray diffractometer (XRD, RIGAKU, D/Max 2500, Japan) with a copper Kα X-ray source with the scanning angle ranging from 5° to 80° (2θ) step size of 0.02° (2θ) and scanning speed of 8°/min. Besides, the microstructure of reduced briquettes was observed by using an Environmental Scanning Electron Microscope (ESEM, FEI QUANTA 200, Holland) equipped with an EDAX energy dispersive X-ray spectroscopy (EDS) detector. ESEM images were recorded in backscattered electron mode operating in a low vacuum of 0.5 Torr and 20 keV. The polished sections were coated with a thin-layer of gold prior to the detection.
Generally, binary basicity is a technical parameter representing standard of limestone dosage in smelting process of iron ores. Unlike iron ore concentrates, the saprolitic laterite ores contains a high proportion of SiO2 and MgO, and a certain amount of Al2O3. Obviously, it is inappropriate to adopt binary basicity in laterite ores smelting. Instead, the quaternary basicity ((CaO+MgO)/(SiO2+Al2O3) mass ratio) should be considered.
Firstly, the saprolitic laterite briquettes with different basicities were roasted at 1300°C for 1 h in 100% CO atmosphere. XRD results of the reduced briquettes are shown in Fig. 3. It is shown that magnesium participated in the generation and transformation of all silicate minerals in the reduction roasting. It indicates that the use of quaternary basicity is reasonable.

XRD patterns of reduced briquettes at 1300°C for 1 h in 100% CO atmosphere.
As seen in Fig. 3, olivine appears firstly during the roasting process at the natural basicity (R=0.5, the basicity of the original laterite ore without addition of flux). With the basicity increasing from 0.5 to 0.8, olivine transforms gradually into monticellite and diopside. Besides, diffraction peaks of diopside disappear at the basicity of 1.4. With further increase of basicity, monticellite also disappears while akermanite and merwinite are observed.
The effect of basicity on the assimilation resultants of CaO is illustrated in Fig. 4. With the addition of CaO, silicates involving olivine (Mg,Fe)2SiO4, diopside CaMgSi2O6, monticellite CaMgSiO4, akermanite Ca2MgSi2O7 and merwinite Ca3Mg(SiO4)2 could be present, depending on the amount of CaO.27,28,29,30)

Effect of basicity on the assimilation resultants of CaO.
The change of characteristic fusion temperatures depends on the mineral composition of reduced products, which is closely associated with the quaternary basicity as shown in Fig. 5. It indicated that the characteristic fusion temperatures (TD, TS, and TF) of the laterite ore decrease initially and then increase, with the basicity increasing from 0.5 (natural basicity) to 1.8.
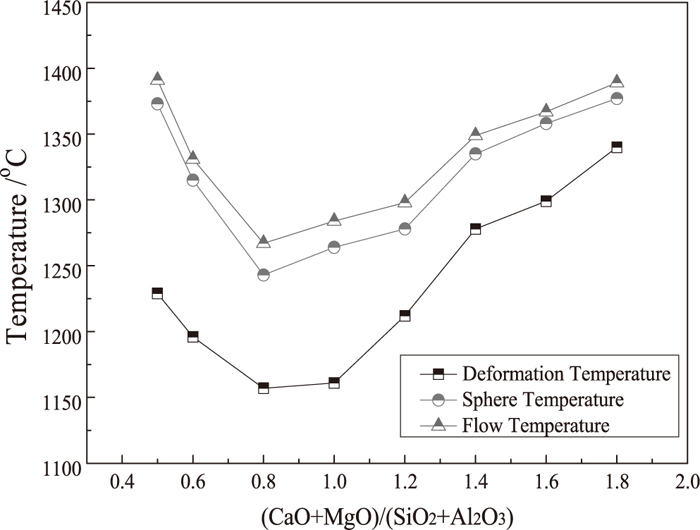
Effect of basicity on characteristic fusion temperatures of laterite ore in 100% CO atmosphere.
As can be seen from Fig. 5, the laterite ore with natural basicity (0.5) is provided with high characteristic fusion temperatures in 100% CO atmosphere, with the corresponding TD, TS, and TF of 1229°C, 1373°C and 1391°C, respectively. By increasing the quaternary basicity, the characteristic fusion temperatures tend to decrease. At the basicity of 0.8, TD, TS, and TF reach a minimum (1157°C, 1243°C and 1267°C, respectively). The results of phase transformation confirmed that the diopside with low melting point of 1391°C is formed at the basicity of 0.8–1.2, leading to lower characteristic fusion temperatures. However, the transitions from diopside and monticellite to refractory silicates akermanite and merwinite contribute to increase of characteristic fusion temperatures. Thus, increasing the basicity by adding an appropriate amount of CaO can lower the melting point temperature over 100°C, improving the formation of liquid phase at lower reduction temperature.
Furthermore, the briquettes with various basicities were roasted in 100% CO atmosphere, and the shapes of reduced briquettes are shown in Fig. 6. As seen from Fig. 5, the deformation temperature (TD) of laterite ore and CaO mixture exceeds 1100°C, hence the briquettes remain its shape after roasting at 1100°C for the given time period (see Fig. 6(A)). While roasting at 1200°C, changes in shapes of briquettes with basicities of 0.6–1.2 take place, and the softening of briquettes is observed (see Fig. 6(B)). Furthermore, by increasing temperature to 1300°C, remarkable fusion occurs in all briquettes (see Fig. 6(C)), particularly those with basicities of 0.8–1.2. Large amounts of liquid phase are generated because the flow temperature (TF) of laterite ore and CaO mixture is lower than 1300°C. As a consequence, adjusting basicity and reduction temperature can effectively increase the quantity of liquid phase.

Appearance shape of reduced briquettes with various basicities in 100% CO atmosphere.
Liquid phase promotes mass transfer and ferronickel particles growth. Microstructures of reduced briquettes with various basicities at 1100–1300°C for 1 h in 100% CO atmosphere are shown in Figs. 7 and 8, respectively. It can be seen from Figs. 7(A) and 7(B) that the structure of raw materials remains after reductive roasting at 1100°C. Tiny ferronickel particles which distribute randomly are observed. With the reduction temperature reaching 1200°C, the structure of materials is destroyed and the ferronickel particles aggregated and grew up (see Figs. 7(C) and 7(D)). Some particle size exceeds 20 μm in the reduced briquettes with basicity of 0.8.
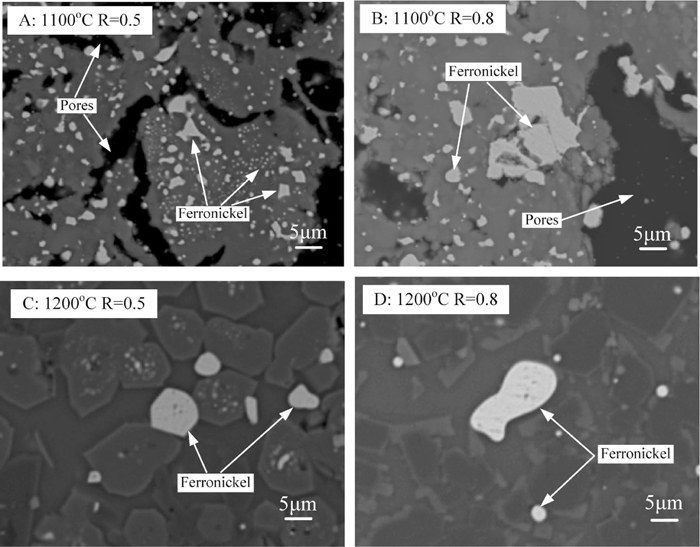
ESEM images of reduced briquettes for 1 h in 100% CO atmosphere.
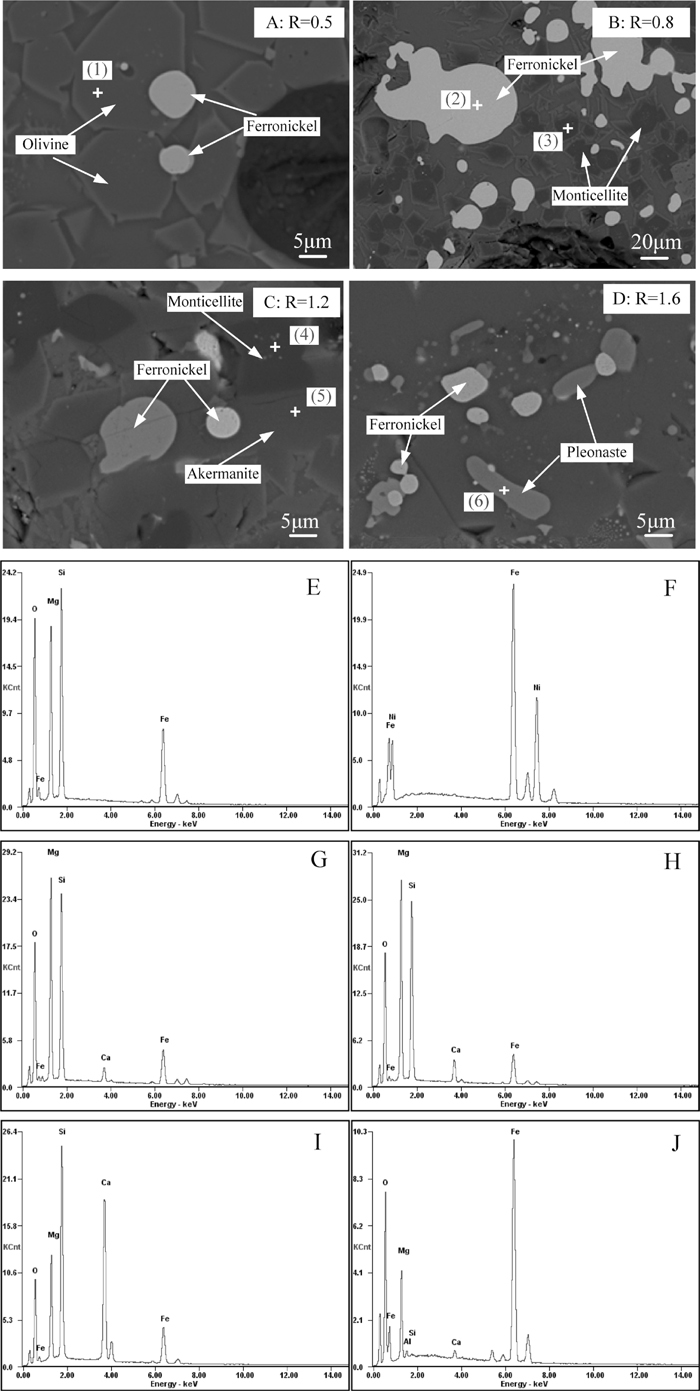
ESEM-EDS images of reduced briquettes at 1300°C for 1 h in 100% CO atmosphere (Figs. 8(E)–8(J) correspond to EDS spectrums of spots 1–6, respectively).
Furthermore, it is evident from Figs. 8(A)–8(D) that the structure of the materials is severely damaged and large amounts of melting phase is formed at 1300°C. The ferronickel particles undergo a remarkable aggregation and growth by mass transfer and interacting through the liquid phase. Some ferronickel particles can grow up to 50 μm at the basicity of 0.8. The EDS spectra of reduced briquettes in Figs. 8(E)–8(J) further verify the phase transformation of silicate minerals from olivine to monticellite and then to akermanite in the basicity range from 0.5 to 1.6.
The average size of ferronickel particles of reduced briquettes was calculated, and the result is shown in Fig. 9. The average size of ferronickel particles increases with temperature from 1100°C to 1300°C (Fig. 9(A)). Besides, the average size of ferronickel particles first increases and then decreases in the basicity range from 0.6 to 1.8 at temperature of 1300°C, and the average particle size is increased to about 31–34 μm at basicity 0.8–1.2 (Fig. 9(B)). From these results, the relationship between the ferronickel particles size and the fusion temperature can be determined: lowering fusion temperature promotes the growth of ferronickel particles. It indicates that the stability and quality of ferronickel alloy production of Krupp-Renn process can be improved by adjusting and optimizing the quaternary basicity of laterite ores.

Average size of ferronickel particles of reduced briquettes for 1 h in 100% CO atmosphere.
In this study, the concept of quaternary basicity of laterite ore is put forward to optimize the limestone dosage for ferronickel production by Krupp-Renn process. The melting characteristics and ferronickel particles growth of laterite ore as the function of quaternary basicity were investigated. The conclusions are obtained as follows:
(1) By adding CaO to adjust basicity from 0.5 to 1.8, characteristic fusion temperatures of the laterite ore first decrease and then increase in 100% CO atmosphere. The characteristic fusion temperatures to about 1300°C at the basicity of 0.8–1.2 ascribed to the generation of diopside with low melting point. When basicity increases to 1.4–1.8, refractory materials akermanite and merwinite appear, and the characteristic fusion temperatures begin to increase.
(2) The formation of the liquid phase is conducive to the growth of ferronickel particles, and ferronickel particles size can be increased by optimizing the quaternary basicity in the reduction process of laterite ores. When the materials are roasted at 1300°C, large amounts of liquid phase are generated in the basicity range of 0.8–1.2 with the lowest characteristic fusion temperatures, resulting in the aggregation of ferronickel particles.
The authors wish to express their thanks to the National Natural Science Foundation of China (Grants 51174230 and 51234008) and the Program for New Century Excellent Talents in University (NCET-11-0515) for financial support of this research. This work was also financially supported by Co-Innovation Center for Clean and Efficient Utilization of Strategic Metal Mineral Resources.